Method for synthesizing cis/trans oxygen and nitrogen heteroatom-containing spiro compound
A technology of spiro compounds and heteroatoms, applied in the field of organic synthesis, can solve the problems of harsh reaction conditions, small expansion range of substrates, long reaction time, etc., and achieve the effects of high yield, environmental friendliness and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
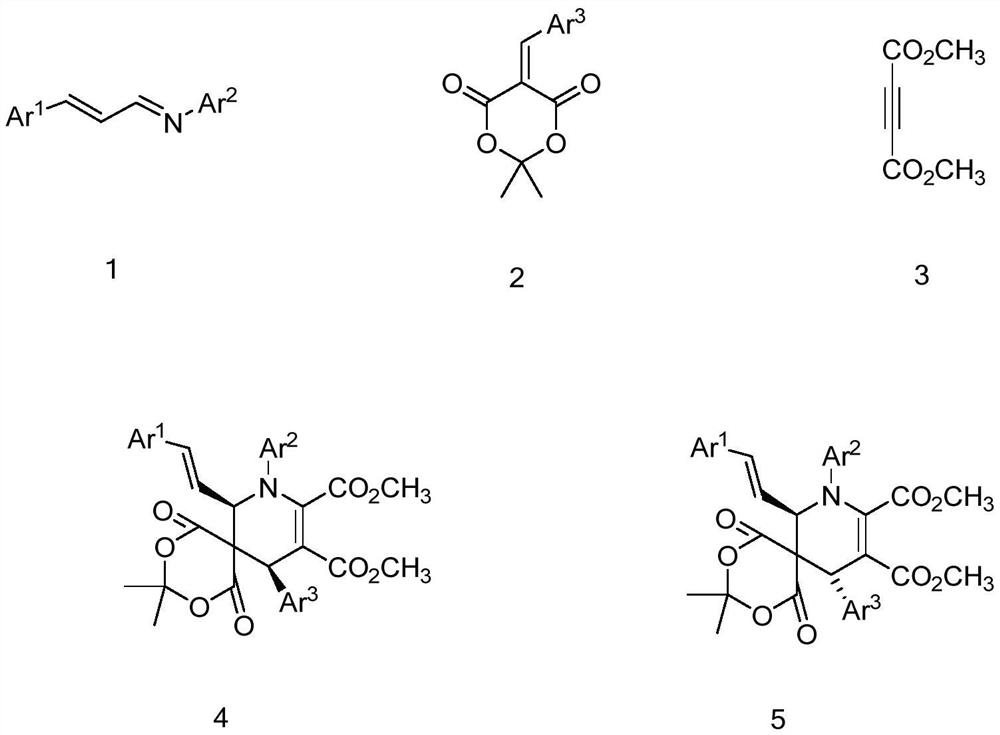
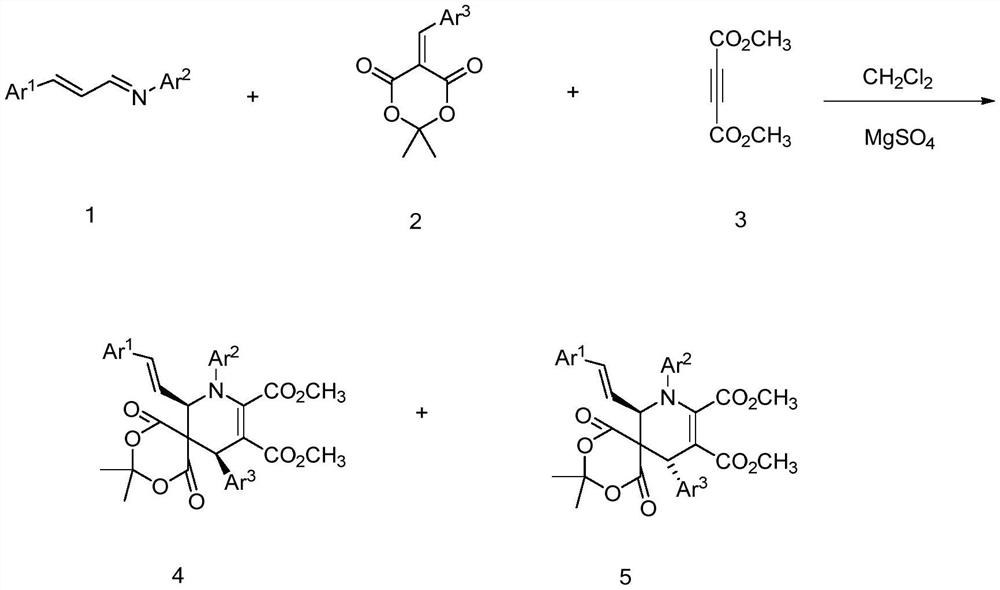
[0024] To prepare cis-dimethyl-8,11-bis(4-methoxyphenyl)-7-((E)-4-methoxystyryl)-3,3-dimethyl -1,5-dioxa-2,4-dioxa-8-azaspiro[5.5]undec-9-ene-9,10-dicarboxylate as an example, its preparation method is as follows:
[0025]
[0026] Add 5 mL of dichloromethane and 0.1800 g (1.5 mmol) of anhydrous magnesium sulfate to a 10 mL Schenk tube, then add 0.2937 g (1.1 mmol) of (1E,2E)-N,3-bis(4-methoxyphenyl) Prop-2-ene-1-imine, 0.2096g (0.8mmol) 5-(4-methoxybenzylidene)-2,2-dimethyl-1,3-dioxane-4,6- Diketone, finally add 0.1420g (1mmol) butynedioic acid dimethyl ester, react at room temperature for 12 hours, remove anhydrous magnesium sulfate by suction filtration after the reaction finishes, evaporate and concentrate the filtrate, utilize a silica gel column to carry out column chromatography purification process, Pure cis-dimethyl-8,11-bis(4-methoxyphenyl)-7-((E)-4-methoxystyryl)-3,3-dimethyl- 1,5-Dioxa-2,4-dioxa-8-azaspiro[5.5]undec-9-ene-9,10-dicarboxylate, isolated in 63% yi...
Embodiment 2
[0029] To prepare trans-dimethyl-8,11-bis(4-methoxyphenyl)-7-((E)-4-methoxystyryl)-3,3-dimethyl -1,5-dioxa-2,4-dioxa-8-azaspiro[5.5]undec-9-ene-9,10-dicarboxylate as an example, its preparation method is as follows:
[0030]
[0031]Purification by column chromatography using a silica gel column in Example 1 to obtain pure trans-dimethyl-8,11-bis(4-methoxyphenyl)-7-((E)-4-methanol Oxystyryl)-3,3-dimethyl-1,5-dioxa-2,4-dioxa-8-azaspiro[5.5]undec-9-ene-9,10 -dicarboxylate, its isolated yield is 30%, and the structural characterization data are as follows:
[0032] 1 H NMR (400MHz, CDCl 3 )δ: 7.28(d, J=9.2Hz, 2H, ArH), 7.24(t, J=8.4Hz, 2H, ArH), 7.04(d, J=7.6Hz, 2H, ArH), 6.89(d, J =8.4Hz,2H,ArH),6.79(d,J=8.8Hz,2H,ArH),6.74(d,J=8.4Hz,2H,ArH),6.36(d,J=15.6Hz,1H,CH) ,5.74(dd,J 1 =16.0Hz,J 2 =9.2Hz,1H,CH), 4.77(d,J=8.8Hz,1H,CH),4.56(s,1H,CH),3.81(s,3H,OCH 3 ),3.75(s,6H,2OCH 3 ),3.55(s,3H,OCH 3 ),3.50(s,3H,OCH 3 ),1.72(s,3H,CH 3 ),1.52(s,3H,CH 3 ); 13 C NMR (100MHz, ...
Embodiment 3
[0034] To prepare cis-dimethyl-11-(4-methoxyphenyl)-7-((E)-4-methoxystyryl)-3,3-dimethyl-1 with the following structural formula, 5-dioxa-8-(p-tolyl)-2,4-dioxa-8-azaspiro[5.5]undec-9-ene-9,10-dicarboxylate as an example, its The preparation method is as follows:
[0035]
[0036] Add 5 mL of dichloromethane and 0.1203 g (1.0 mmol) of anhydrous magnesium sulfate to a 10 mL Schenk tube, then add 0.3012 g (1.2 mmol) of (1E,2E)-3-(4-methoxyphenyl)-N- (p-tolyl)prop-2-en-1-imine, 0.2620g (1.0mmol) 5-(4-methoxybenzylidene)-2,2-dimethyl-1,3-dioxane -4,6-diketone, finally add 0.1420g (1mmol) dimethyl butynedioate, react at room temperature for 12 hours, remove anhydrous magnesium sulfate by suction filtration after the reaction is completed, evaporate and concentrate the filtrate, and use a silica gel column for column Chromatographic purification treatment, you can get cis-dimethyl-11-(4-methoxyphenyl)-7-((E)-4-methoxystyryl)-3,3-dimethyl -1,5-dioxa-8-(p-tolyl)-2,4-dioxa-8-azasp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com