Recombinant porcine IL-29 fusion protein and preparation method and application thereof
A fusion protein, pil-29-fc technology, applied in the field of recombinant porcine IL-29 fusion protein and its preparation, can solve the problems of lack, increase the difficulty of operation, insufficient yield, etc., and achieve the advantages of reducing side effects and reducing the number of administrations. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Construction of pIL-29Fc and pIL-29-PSA fusion protein recombinant cell lines
[0044] Nucleotide search of porcine IL-29, porcine Fc and porcine PSA in UniProt and GenBank databases or amino acid sequence. According to the codon preference of hamsters, pIL-29-Fc and pIL-29-PSA were codon-optimized, and related nucleotides were artificially synthesized. The codon-optimized pIL-29-Fc nucleotide sequence is shown in SEQ ID NO Shown in: 1, the codon-optimized pIL-29-PSA nucleotide sequence is shown in SEQ ID NO: 3. The synthetic pIL-29-Fc and pIL-29-PSA nucleotides were respectively constructed in the pcDNA3.1 vector to obtain the recombinant expression vectors pcDNA3.1-pIL-29-Fc and pcDNA3.1-pIL-29-PSA. After the pcDNA3.1-pIL-29-Fc recombinant vector was linearized, it was electroporated into CHO cells, and the pIL-29-Fc stably transfected cell line pIL-29-Fc / CHO was obtained after pressurized screening; the pcDNA3. After the 1-pIL-29-PSA recombinant vector ...
Embodiment 2
[0045] Example 2 Purification of pIL-29Fc and pIL-29-PSA fusion protein
[0046] Recombinant cells pIL-29-Fc / CHO and pIL-29-PSA / CHO were fermented and cultured in a fermenter, and the fermentation broth was first passed through two-stage deep filter membrane bag to remove cells and cell debris, and then filtered with a 0.22 μm filter membrane , to obtain a clarified fermentation broth. pIL-29Fc and pIL-29-PSA were purified using the following methods, respectively.
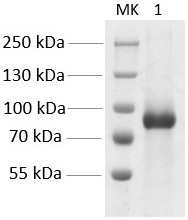
[0047] Purification of pIL-29Fc: The fermentation broth was first purified by affinity chromatography Protein A (MabSelect SuReTM, GE Healthcare): first equilibrated to the baseline with an equilibrium solution (50 mM glycine, 0.15 M NaCl, pH 7.2), and then with the eluent ( 50 mM glycine, pH 3.0) and collect the eluate. The protein A collected solution was purified by anion exchange Capto Q (GE Healthcare) column chromatography: the collected solution was adjusted to pH 8.0 with 1M NaOH, equilibrated to baselin...
Embodiment 3
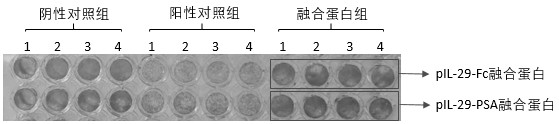
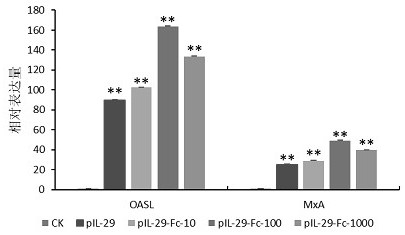
[0049] Example 3 Determination of biological activity of pIL-29Fc and pIL-29-PSA fusion protein
[0050] The titers of pIL-29-Fc and pIL-29-PSA were determined by cytopathic inhibition method. Dilute well-grown MDBK (bovine kidney cell) cell suspension by 2×10 5 The density of cells / mL was inoculated in a 96-well cell culture plate, 100 μL / well, and cultured overnight at 37°C in a 5% CO2 cell incubator. Dilute pIL-29, pIL-29-Fc and pIL-29-PSA in 4-fold gradients with 2% FBS-DMEM respectively, set 8 replicates for each gradient, add 100 μL protein / well, and store at 37°C, 5% CO2 Continue culturing for 24 h in the cell culture incubator. The cell culture medium was discarded, and the cells were infected with 100 TCID50 of VSV per well, 24h after infection. Discard the culture medium, add 50 μL of crystal violet staining solution to each well and stain at room temperature for 30 min, wash away the staining solution with running water, add 100 μL of destaining solution to each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com