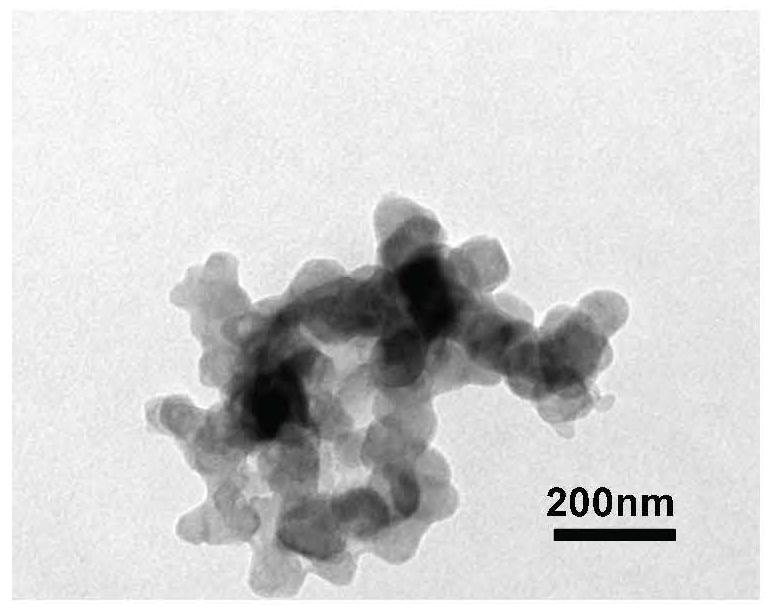
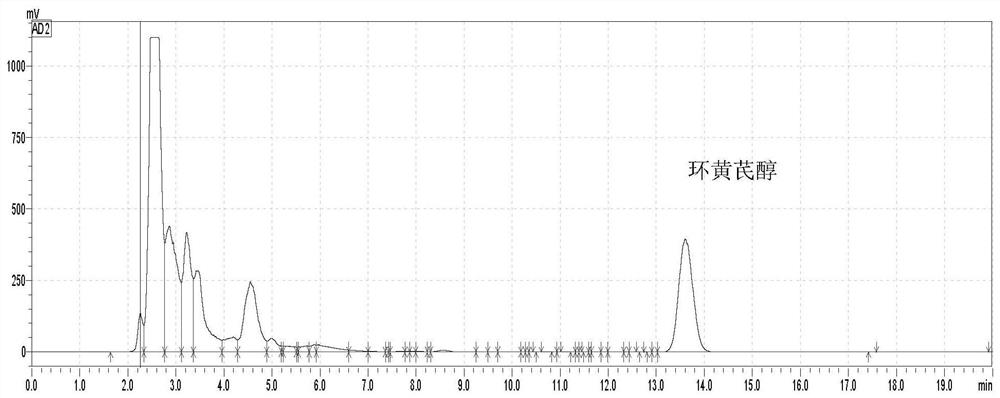
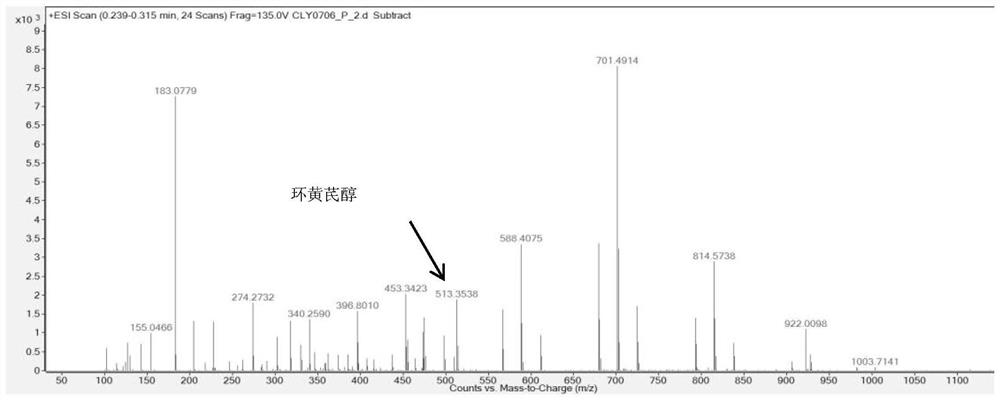
Method for preparing cycloastragenol by catalyzing astragaloside through co-immobilized double enzymes
A technology of astragaloside IV and cycloastragenol, which is applied in the field of catalysis and can solve the problems of limiting production efficiency, increasing production costs, and the inability to realize recycling and reuse of free enzymes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Prepare a phosphate buffer solution by mixing disodium hydrogen phosphate with a concentration of 0.2mol / L and a volume of 19.15mL and citric acid with a concentration of 0.1mol / L and a volume of 0.85mL to prepare a phosphate buffer solution with a concentration of 1mg / mL and a volume of 60μL β-glucosidase was added to 1 mL of phosphate buffered saline solution (take part of the prepared phosphate buffered saline solution). Then add Fe at a concentration of 5 mg / mL in a volume of 100 μL 3 o 4 Mix the solution, then add a copper chloride solution with a concentration of 180mM and a volume of 100μL for reaction, let it stand at room temperature for 24 hours, collect the precipitate with a magnet, and mix the collected precipitate with 1mg / mL xylosidase and phosphate buffer solution (same composition as the above-mentioned phosphate buffer solution) for the reaction, the volumes of xylosidase and phosphate buffer solution were 60 μL and 1.2 mL respectively. After standin...
Embodiment 2
[0048] Prepare a phosphate buffer solution by mixing disodium hydrogen phosphate with a concentration of 0.15 mol / L and a volume of 23.15 mL and citric acid with a concentration of 0.07 mol / L and a volume of 1.85 mL to prepare a phosphate buffer solution with a concentration of 0.5 mg / mL and a volume of 40 μL β-glucosidase was added to 0.8mL phosphate buffer solution (take part of the prepared phosphate buffer solution). Then add Fe at a concentration of 3 mg / mL in a volume of 80 μL 3 o 4 Mix the solution evenly, then add a copper chloride solution with a concentration of 150mM and a volume of 80μL for reaction, let it stand at room temperature for 18 hours, collect the precipitate with a magnet, and mix the collected precipitate with 0.5mg / mL xylosidase, phosphate buffered saline Solution (same composition as the above-mentioned phosphate buffer solution) was reacted, and the volumes of xylosidase and phosphate buffer solution were 40 μL and 1.0 mL, respectively. After stan...
Embodiment 3
[0052] Prepare a phosphate buffer solution by mixing disodium hydrogen phosphate with a concentration of 0.2mol / L and a volume of 19.15mL and citric acid with a concentration of 0.1mol / L and a volume of 0.85mL to prepare a phosphate buffer solution with a concentration of 2.5mg / mL and a volume of 80μL β-glucosidase was added to 1.6mL phosphate buffer solution (take part of the prepared phosphate buffer solution). Then add Fe at a concentration of 8 mg / mL in a volume of 120 μL 3 o 4 The solution was mixed evenly, and then a copper chloride solution with a concentration of 250 mM and a volume of 120 μL was added for reaction. After standing at room temperature for 24 hours, the precipitate was collected with a magnet, and the collected precipitate was mixed with 2.5 mg / mL xylosidase, phosphate buffered saline Solution (same composition as the above-mentioned phosphate buffer solution) was reacted, and the volumes of xylosidase and phosphate buffer solution were 80 μL and 2 mL, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com