Sterile detection method of voriconazole for injection
A voriconazole and detection method technology, which is applied in the field of sterility detection of voriconazole for injection, can solve the problems of reduced detection rate, excessive flushing volume, and high detection cost, so as to reduce antibacterial performance, save detection cost, and improve accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The specification of the voriconazole for injection detected in this embodiment is 0.2 g / bottle, and the batch number of the voriconazole is V190701.
[0046]Specifically, the sterility testing process of the voriconazole for injection is as follows:
[0047] 1 Preparation and counting of bacteria solution
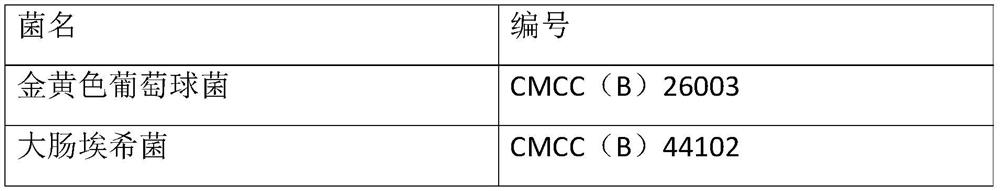
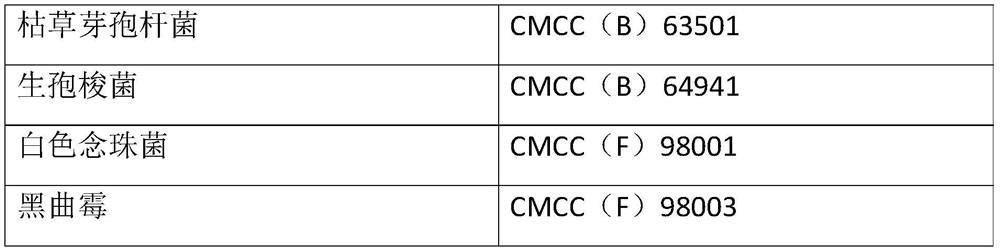
[0048] Select bacteria powders containing six kinds of bacteria including Escherichia coli, Staphylococcus aureus, Clostridium sporogenes, Bacillus subtilis, Candida albicans and Aspergillus niger, the concentration of each bacteria powder is 110-1100cfu, before use First dissolve the corresponding bacterial powder with 1.1ml of the supporting solvent and mix well, so that the concentration of the bacteria is 100-1000cfu / ml. When using, add 0.1ml of the bacteria liquid, that is, the concentration of the added bacteria is 10-100cfu / 0.1ml. At the same time, take 0.1ml of Escherichia coli, Staphylococcus aureus, and Bacillus subtilis into tryptone soy agar medium, and...
Embodiment 2
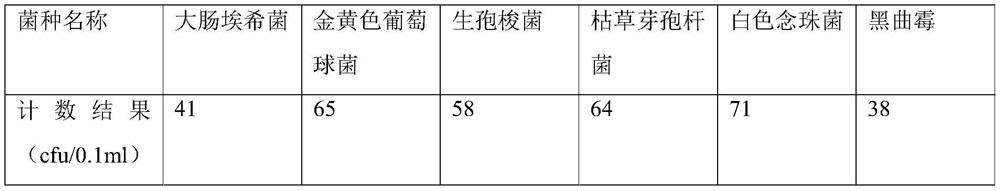
[0062] Examples 2-3 were tested according to the sterility detection method provided in Example 1, the difference being that the batch numbers of voriconazole for injection used were different, specifically, the batch number of voriconazole used in Example 2 was V190801, and the batch number of voriconazole used in Example 3 was V190801. The batch number is V190802. The difference between Example 4 and Example 1 is that the batch numbers of voriconazole for injection used are different V200101, V191201, and V191202, and both the flushing solution and the dissolving solution are 0.1% sulfobutylbeta-cyclodextrin sodium solution. And the enumeration result of each bacterial species in the bacterium liquid prepared in embodiment 2-embodiment 4 sees the following table:
[0063]
[0064] The sterility test result of embodiment 2 is as follows:
[0065]
[0066] Note: "—" indicates clarification without growth; "+" indicates good growth. The test article grew aseptically wit...
Embodiment 3
[0067] The sterility test result of embodiment 3 is as follows:
[0068]
[0069] Note: "—" indicates clarification without growth; "+" indicates good growth. The test article grew aseptically within 14 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com