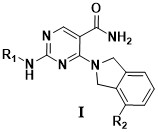
2-amino-4-(isoindoline-2-yl) pyrimidine-5-formamide derivative, preparation method and application thereof
A substance, phenyl technology, applied in the field of preparation of 2-amino-4-pyrimidine-5-carboxamide derivatives, can solve problems such as no effective method, and achieve the effect of good anti-tumor or immune disease pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
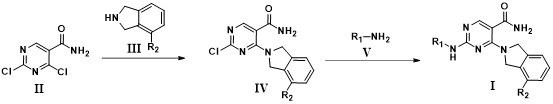
Examples
Embodiment 1
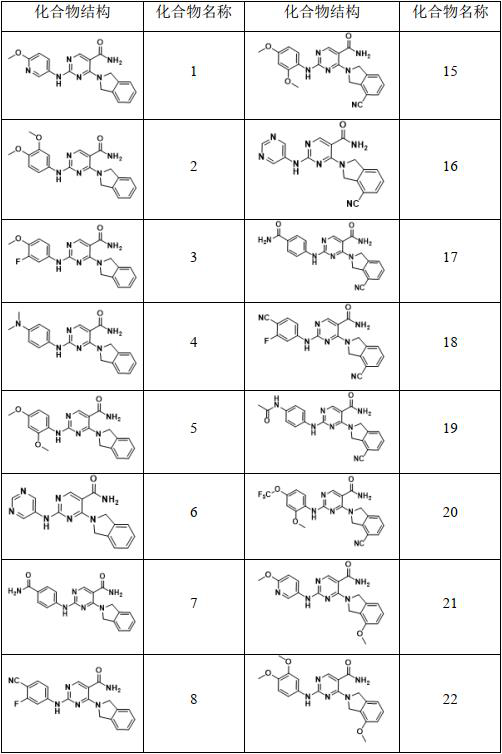
[0048] 4-(isoindoline 2-yl)-2-((6-methoxypyridin-3-yl)amino)pyrimidine-5-carboxamide
[0049]
[0050] Synthesis of Intermediate 1c:
[0051] Dissolve compound 1a (19.1g, 100.0mmol), compound 1b (11.9g, 100.0mmol), cesium carbonate (39.0g, 120.0mmol) in DMF (500ml), raise the temperature to 80°C and stir for 8 hours, then detect the reaction by TLC After the reaction was completed, the reaction was quenched with water (300ml), then extracted twice with ethyl acetate (500ml), the organic layer was concentrated, and separated by column chromatography to obtain 20.4g of a light yellow solid (compound 1c), with a yield of 74.5%.
[0052] Synthesis of compound 1:
[0053] Compound 1c (2.7g, 10.0mmol), compound 1d (1.2g, 10.0mmol), Pd(dppf) 2 Cl 2 (732 mg, 1.0 mmol), K 2 CO 3 (1.7g, 12.0mmol) was dissolved in DMF (50ml), heated to 90°C and reacted for 8 hours, after the reaction was completed, water (50ml) was added to quench the reaction, ethyl acetate (100ml) was extracted...
Embodiment 2
[0055] 2-((3,4-Dimethoxyphenyl)amino)-4-(isoindoline-2-yl)pyrimidine-5-carboxamide
[0056]
[0057] The synthesis of intermediate 1c was carried out as in Example 1.
[0058] Synthesis of compound 2:
[0059] Compound 1c (2.7g, 10.0mmol), compound 2a (1.5g, 10.0mmol), Pd(dppf) 2 Cl 2 (732 mg, 1.0 mmol), K 2 CO 3 (1.7g, 12.0mmol) was dissolved in DMF (50ml), heated to 90°C and reacted for 8 hours, after the reaction was completed, water (50ml) was added to quench the reaction, ethyl acetate (100ml) was extracted twice, the organic layer was dried, filtered, Column chromatography separated to obtain 2.1 g of an off-white solid (compound 2), with a yield of 53.7%, ESI(+) m / z=392.2.
Embodiment 3
[0061] 2-((3-fluoro-4-methoxyphenyl)amino)-4-(isoindoline-2-yl)pyrimidine-5-carboxamide
[0062]
[0063] The synthesis of intermediate 1c was carried out as in Example 1.
[0064] Synthesis of compound 3:
[0065] Compound 1c (2.7g, 10.0mmol), compound 3a (1.4g, 10.0mmol), Pd(dppf) 2 Cl 2 (732 mg, 1.0 mmol), K 2 CO 3 (1.7g, 12.0mmol) was dissolved in DMF (50ml), heated to 90°C and reacted for 8 hours, after the reaction was completed, water (50ml) was added to quench the reaction, ethyl acetate (100ml) was extracted twice, the organic layer was dried, filtered, Column chromatography separated to obtain 2.5 g of off-white solid (Compound 3), with a yield of 66.0%, ESI(+) m / z=380.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com