Method for preparing polyester by catalyzing cyclic anhydride/epoxide ring opening alternate copolymerization with potassium acetate
A technology of potassium acetate catalyzing cyclic anhydrides and epoxides, which is applied in the field of polyester preparation, can solve the problems of high biological toxicity and high price, and achieve the effects of low biological toxicity, low price and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142]
[0143] The preparation method of polyphthalate-cyclohexanediol in the present embodiment comprises the following steps:
[0144] (1) Under an inert atmosphere, add 15 μmol of potassium acetate catalyst, 1500 μmol of phthalic anhydride and 7500 μmol of cyclohexene oxide into a dry 15mL reactor, stir for 5 minutes, stabilize the reaction temperature to 110°C, and vigorously stir Lower polymerization reaction 6 hours;
[0145] (2) After the polymerization is completed, cool the reactor to room temperature, and pour the reaction system in the reactor into 500 mL of methanol for sedimentation. Then, it is filtered, washed and vacuum-dried to obtain polyphthalate-cyclohexanediol ester.
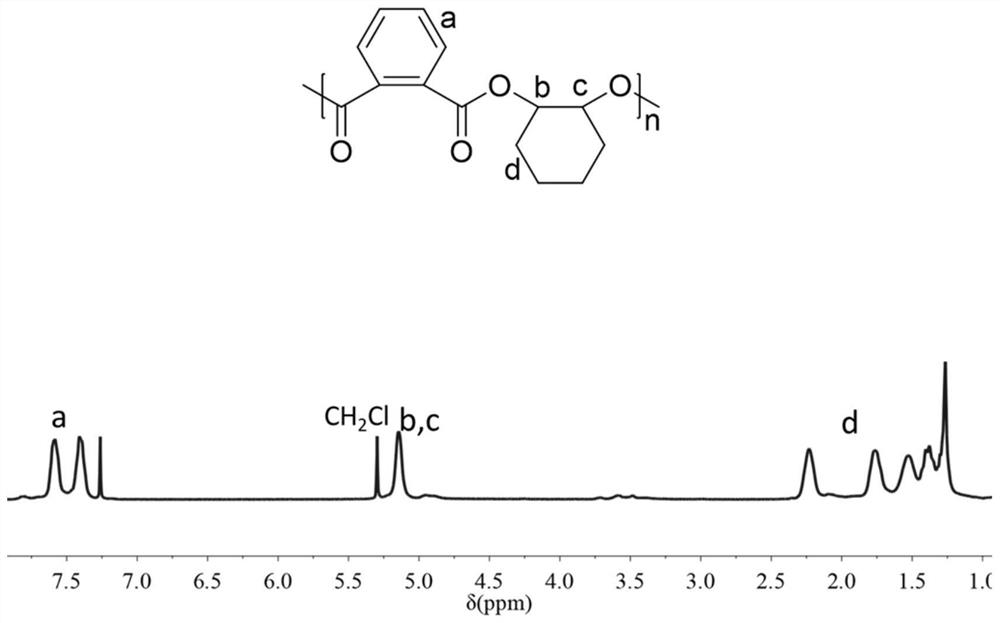
[0146] The polymerization time was 6 hours, and the conversion rate of phthalic anhydride monomer reached 100%. The product obtained above was subjected to GPC analysis and NMR analysis. Such as figure 1 As shown, the product obtained in Example 1 of the present invention is a polyes...
Embodiment 2
[0148]
[0149] The preparation method of poly-1,2-propylene glycol phthalate in the present embodiment comprises the following steps:
[0150] (1) Under an inert atmosphere, add 15 μmol of potassium acetate catalyst, 1500 μmol of phthalic anhydride and 7500 μmol of propylene oxide into a dry 15mL reactor, stir for 5 minutes, stabilize the reaction temperature to 110°C, and Polymerization for 8 hours;
[0151] (2) After the polymerization is completed, cool the reactor to room temperature, and pour the reaction system in the reactor into 500 mL of methanol for sedimentation. Then it is filtered, washed and vacuum-dried to obtain poly-1,2-propylene glycol phthalate.
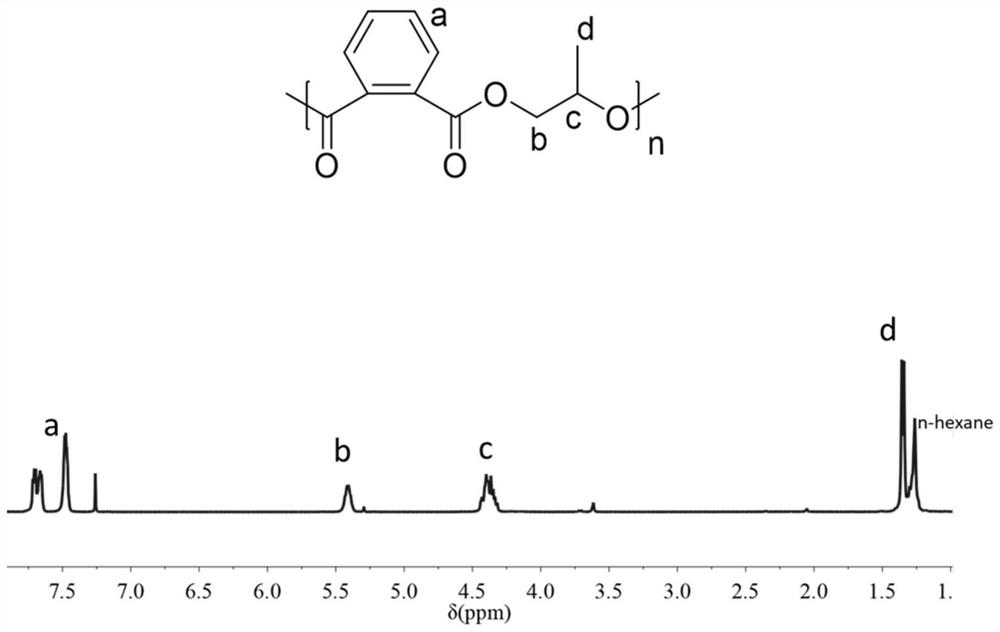
[0152] The polymerization time was 8 hours, and the conversion rate of phthalic anhydride monomer reached 100%. The product obtained above was subjected to GPC analysis and NMR analysis. Such as figure 2 As shown, the product obtained in Example 2 of the present invention is a polyester having a structure ...
Embodiment 3
[0154]
[0155] The preparation method of poly-1,2-propylene glycol phthalate in the present embodiment comprises the following steps:
[0156](1) In an inert atmosphere, add 15 μmol potassium acetate catalyst, 15000 μmol phthalic anhydride and 75000 μmol propylene oxide into a dry 15 mL reactor, stir for 5 minutes, stabilize the reaction temperature to 110 ° C, and Polymerization for 72 hours;
[0157] (2) After the polymerization is completed, cool the reactor to room temperature, and pour the reaction system in the reactor into 1000 mL of methanol for sedimentation. Then it is filtered, washed and vacuum-dried to obtain poly-1,2-propylene glycol phthalate.
[0158] The polymerization time was 72 hours, and the conversion rate of phthalic anhydride monomer reached 100%. The product obtained above was subjected to GPC analysis and NMR analysis. Such as figure 2 As shown, the product obtained in Example 3 of the present invention is a polyester having a structure of fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com