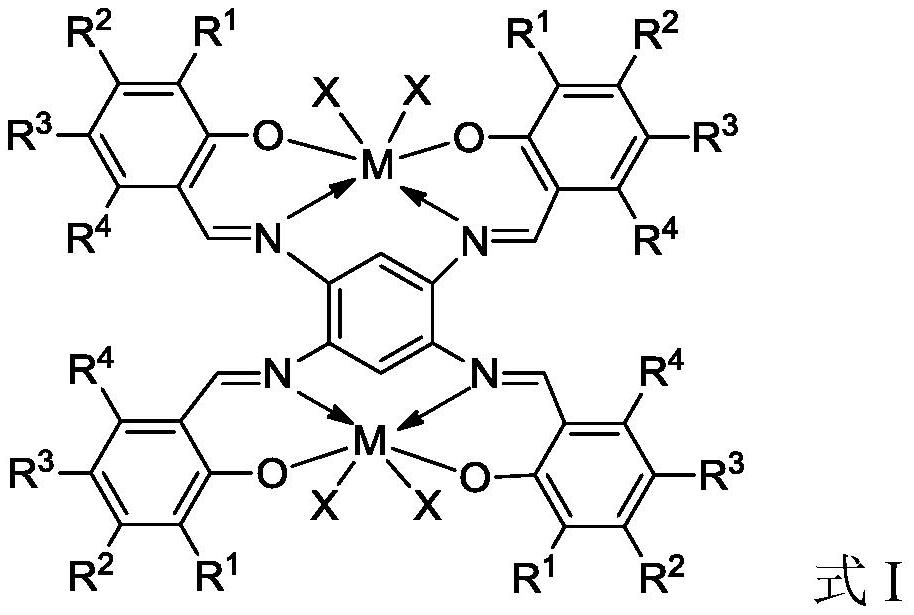
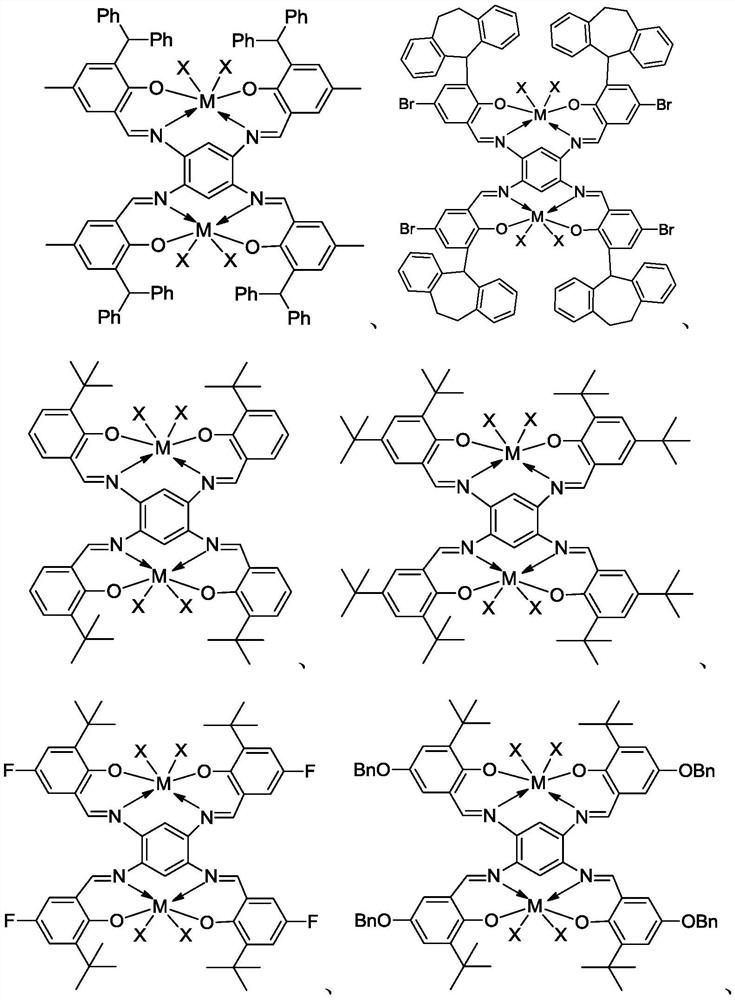
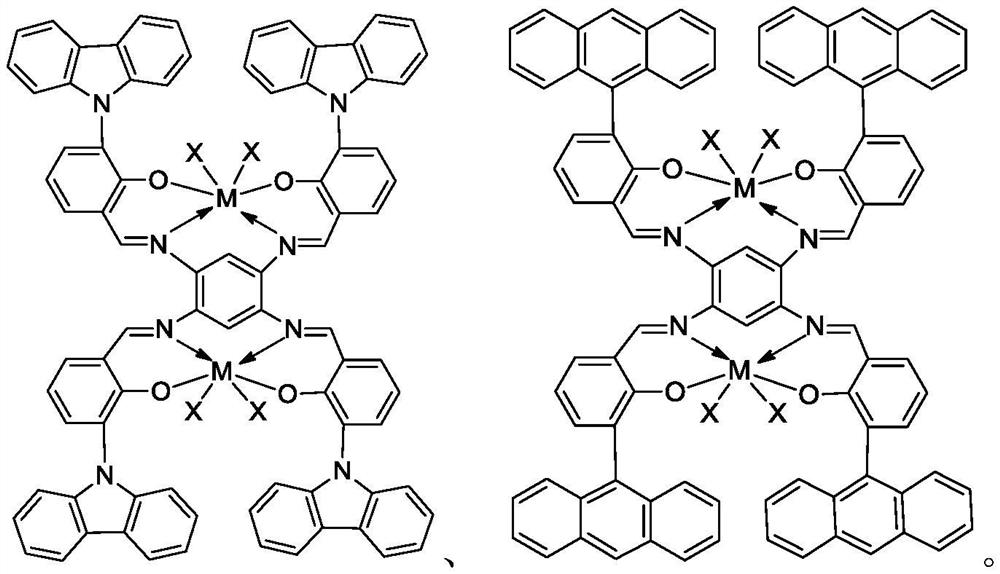
Bimetal complexes of phenoxy imine ligand framework, preparation method and application
A technology of phenoxyimine and complexes, which is applied in the field of bimetallic complexes, can solve the problems of catalyst thermal stability that needs to be improved, polymer molecular weight is low, and it is difficult to meet application requirements, so as to achieve good catalytic activity and thermal stability , The effect of broadening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Under a nitrogen atmosphere, 6.81g of 2-hydroxy-5-methylbenzaldehyde (50mmol, 1.0eq.) and 9.21g of diphenylmethanol (50mmol, 1.0eq.) were dissolved in 150mL of dichloromethane, and slowly added 1.10g of Tin bromide (2.5mmol, 0.05eq.), stirred at room temperature for 8h. Add 10 mL of saturated aqueous sodium bicarbonate solution to quench the reaction, extract with dichloromethane, wash with saturated brine, combine the organic phases, dry over anhydrous sodium sulfate, concentrate the filtrate and purify by silica gel column chromatography (petroleum ether: ethyl acetate = 100: 1 (v / v)), to obtain 14.15 g of white solid, which was denoted as compound 1, and the yield was 93.6%.
[0090] The NMR structure confirmation data of compound 1 are as follows:
[0091] 1 H NMR (CDCl 3 ,400MHz,TMS):δ12.03(s,1H),10.18(s,1H),7.39(s,1H),7.29(t,J=8.0Hz,4H),7.20–7.18(m,3H), 7.13(d, J=8.0Hz, 4H), 5.46(s, 1H), 2.35(s, 3H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ190.0,156.7,142.4,136.3,131...
Embodiment 2
[0093] Under a nitrogen atmosphere, 2.84g of 1,2,4,5-benzenetetramine tetrahydrochloride (10mmol, 1.0eq.) and 13.30g of compound 1 (44mmol, 4.4eq.) were dissolved in 100mL of toluene, and 0.38g p-toluenesulfonic acid (2mmol, 0.2eq.), heated under reflux for 12h. The reaction solution was concentrated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 200:1 (v / v)) to obtain 9.99 g of a white solid, which was designated as compound 2, with a yield of 78.3%.
[0094] The NMR structure confirmation data of compound 2 are as follows:
[0095] 1 H NMR (CDCl 3 ,400MHz,TMS):δ11.93(s,4H),8.94(s,4H),7.47(s,4H),7.29(t,J=8.0Hz,16H),7.20(t,J=8.0Hz, 8H), 7.13(d, J=8.0Hz, 16H), 6.95(s, 4H), 6.75(s, 2H), 5.46(s, 4H), 2.35(s, 12H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ159.0,156.0,143.5,142.4,132.8,130.6,129.2,128.2,127.2,125.2,118.4,117.9,49.3,21.6.
Embodiment 3
[0097] In the glove box, dissolve 6.38g of compound 2 (5mmol, 1.0eq.) in 50mL of dry toluene, slowly add 11mL, 2mol / L n-butyllithium (22mmol, 4.4eq.) dropwise, react at room temperature for 1h, and drain Toluene, add 20mL of dry n-hexane, stir for 15min and let stand, filter and wash with dry n-hexane, add 50mL of dry toluene to the filter residue to dissolve, and add 4.98g of TiCl 4 (THF) 2 (15mmol, 3.0eq.), heated to reflux for 8h, drained the toluene after the reaction, added 20mL of dry n-hexane, stirred for 15min and allowed to stand, filtered and washed with dry n-hexane, the filtrate was drained, then added 30mL of dry toluene, filtered The filtrate was collected, and the solvent was drained to obtain 3.95 g of a dark red solid, which was recorded as compound 3, and the yield was 52.4%.
[0098] The NMR structure confirmation data of compound 3 are as follows:
[0099] 1 H NMR (CDCl 3 ,400MHz,TMS):δ8.39(s,4H),7.46(s,4H),7.29(t,J=8.0Hz,16H),7.20(t,J=8.0Hz,8H),7.13(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com