Method for catalyzing hydroboronation of aza-aromatic ring compound by using triazole salt and conjugated base thereof
A nitrogen-heteroaromatic ring and conjugate base technology, applied in the field of organic synthesis, can solve the problems of metal residue and high cost, and achieve the effect of good yield and less catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the preparation of triazole salt conjugate base system
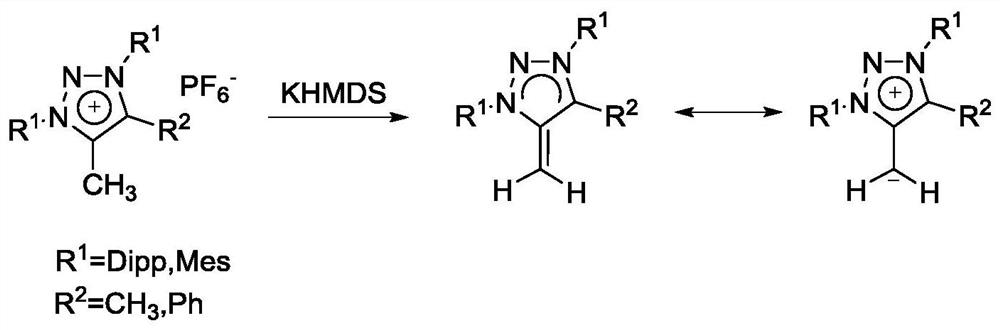
[0036] according to figure 1 The flow chart shown prepares the triazolium salt conjugate base system. The specific operation is as follows:
[0037] Take the triazole salt (R 1 for Mes, R 2 for CH 3 ) (triazole salts were synthesized from known methods) (References: Bouffard, J.; Keitz, B.K.; Tonner, R.; Guisado-Barrios, G.; Frenking, G.; Groubbs, R.H.; Bertrand, G. , Organometallics 2011,30,2617-2627) 0.5mmol, and KHMDS solid 0.55mmol, under the protection of nitrogen, add THF 5ml at -78°C, react at low temperature for 1h, remove the solvent in vacuo, extract with toluene in the glove box, and drain the toluene to obtain the product.
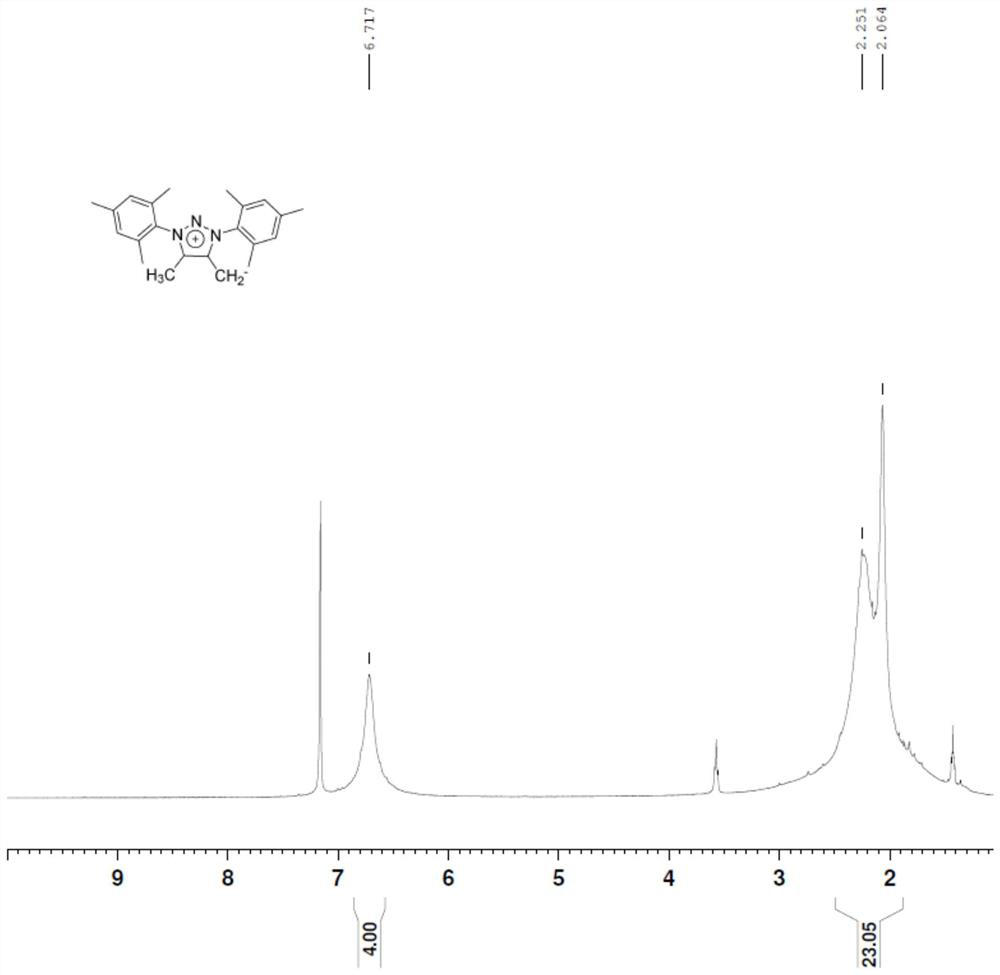
[0038] figure 2 Proton nuclear magnetic resonance spectrum (1H-NMR) for the prepared triazolium salt conjugate base system;
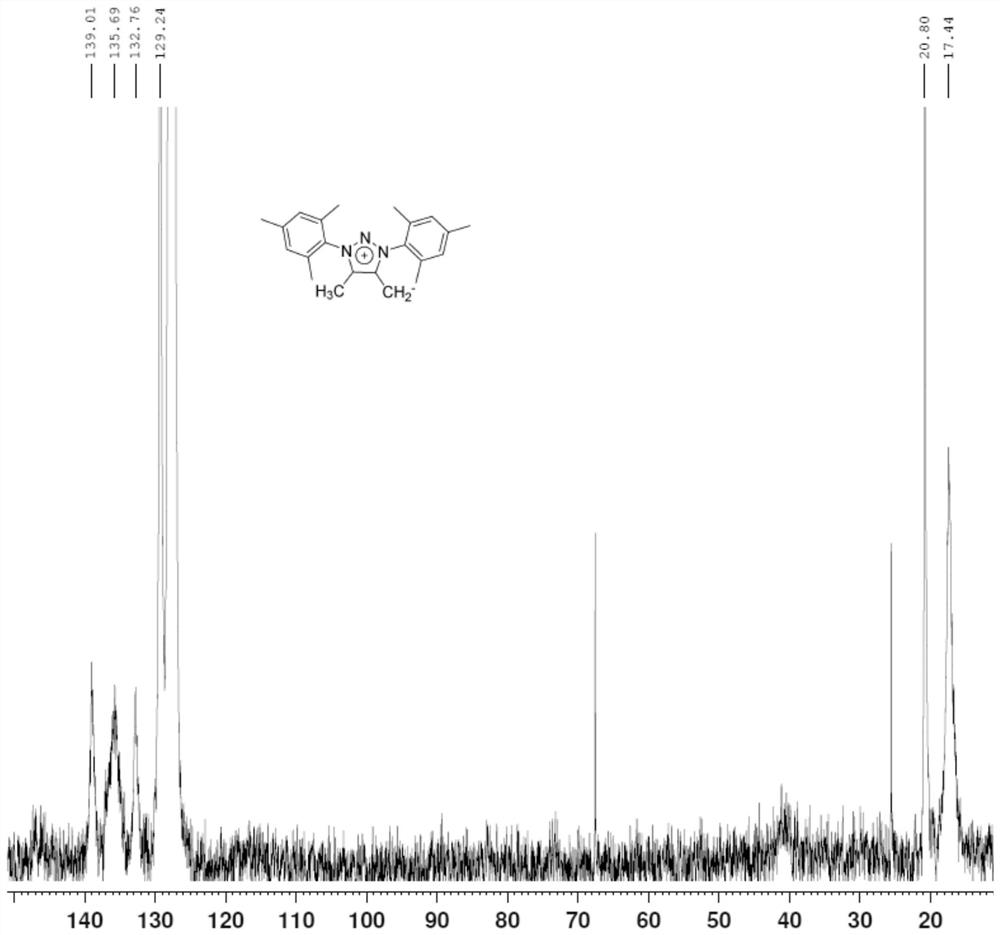
[0039] image 3 It is the carbon nuclear magnetic resonance spectrum (13C-NMR) of the prepared triazolium salt conjugate base system....
Embodiment 2
[0042] Weigh 0.004mmol of the catalyst prepared in Example 1 above, 0.2mmol of pyrimidine, 0.44mmol of HBpin, and 0.7ml of deuterated benzene in a glove box, and react at 70°C for 12h. Drain the solvent to obtain the final product directly, and directly characterize it by NMR. Yield >99%.
[0043] Figure 4 It is the proton nuclear magnetic resonance spectrum (1H-NMR) of the pyrimidine hydroboration product obtained.
[0044] Figure 5 It is the carbon nuclear magnetic resonance spectrum (13C-NMR) of the pyrimidine hydroboration product obtained.
[0045] It was confirmed that the final product was the target product.
Embodiment 3
[0047] In a glove box, 0.004 mmol of the catalyst prepared in Example 1 above, 0.2 mmol of acridine, 0.22 mmol of HBpin, and 0.7 ml of deuterated benzene were weighed, and reacted at 70°C for 12 hours. No need for post-processing, direct NMR characterization, yield >99%.
[0048] Image 6 The proton nuclear magnetic resonance spectrum (1H-NMR) of the acridine hydroboration product prepared in the present invention.
[0049] Figure 7 It is the carbon nuclear magnetic resonance spectrum (13C-NMR) of the acridine hydroboration product prepared in the present invention.
[0050] It was confirmed that the final product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com