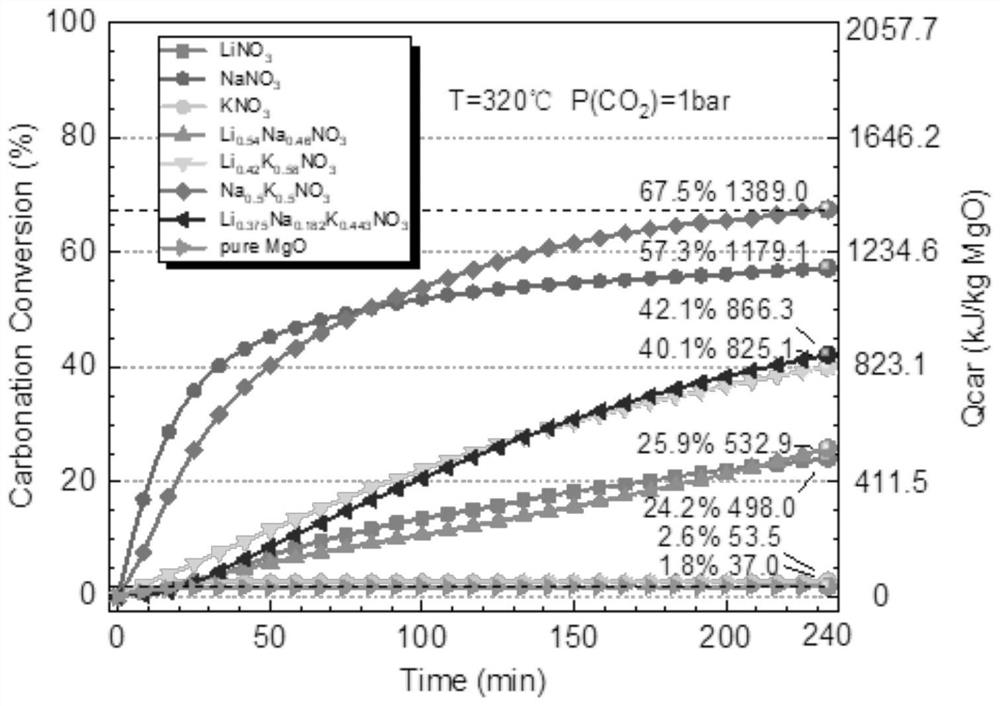
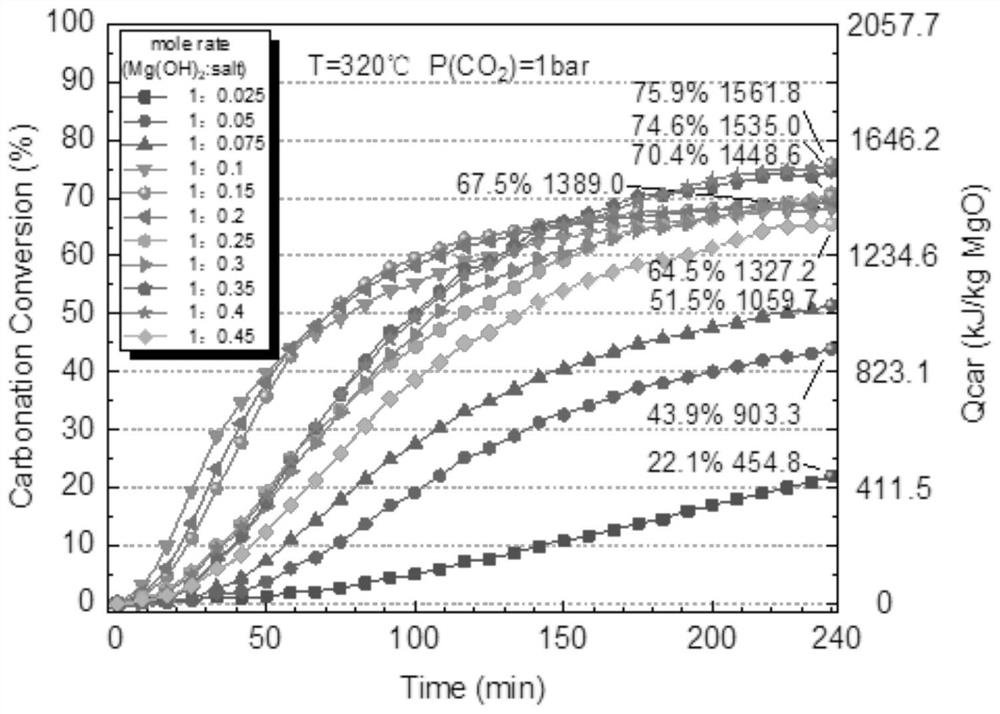
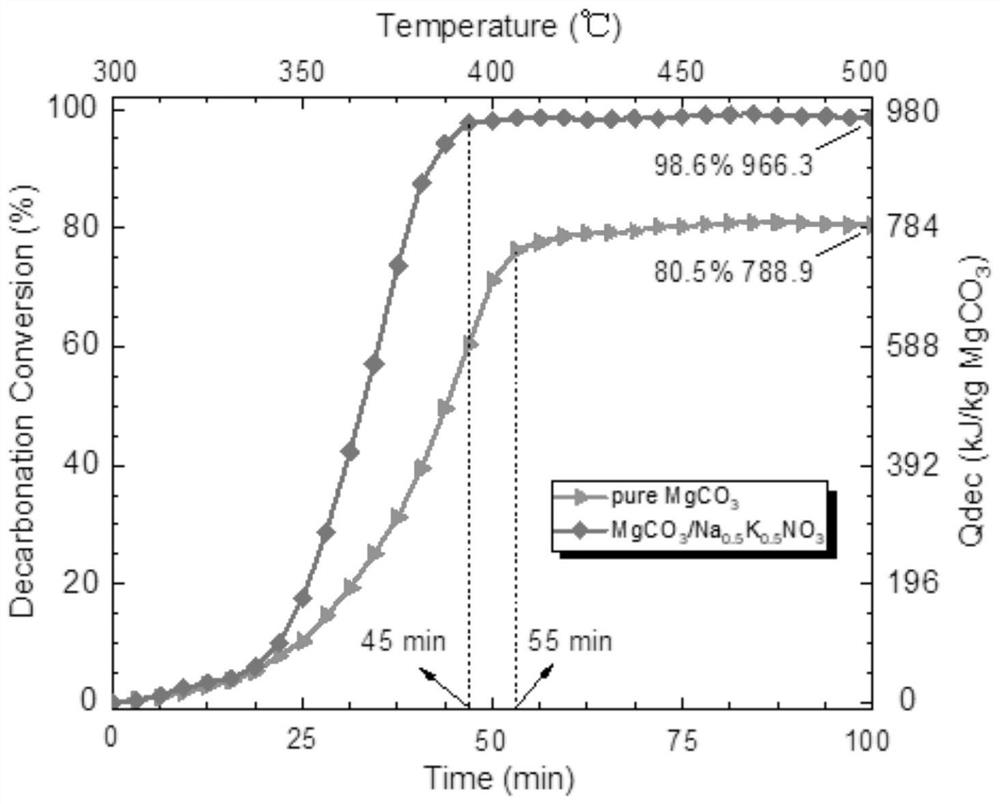
Chemical heat storage and release material with high heat storage and release density and preparation method thereof
A technology for storing and releasing heat and density, which is applied in the direction of heat exchange materials, chemical instruments and methods, etc. It can solve the problems of reducing the specific surface area of solid particles, slow reaction rate, and zero exothermic density, so as to improve the exothermic reaction rate. , The effect of improved kinetic performance and high storage/release heat density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Weigh 0.4250g NaNO 3 (analytical pure, Sinopharm Chemical Reagent Co., Ltd.) and 0.5055g KNO 3 (analytical pure, Sinopharm Group Chemical Reagent Co., Ltd.) was dissolved in a beaker filled with 50ml deionized water, and 5.8320g Mg(OH) was added thereto 2 (super pure ≥ 99%, Sinopharm Chemical Reagent Co., Ltd.) and 0.6008g nanometer powder SiO 2 (analytical grade, Sinopharm Chemical Reagent Co., Ltd.), and stirred in an ultrasonic stirrer for 20 min in a water bath heating environment at 60°C.
[0042] (2) Place the above-mentioned beaker containing the mixed solution on a magnetic stirrer and heat it at a speed of 100 rpm until the solution evaporates to dryness. The evaporated material was placed in a vacuum oven and dried at 80° C. for 12 hours.
[0043] (3) The dried material was dry-milled for 8 hours with a planetary high-speed ball mill (QS92, Nanjing Nanda Instrument Factory). Only use 1 spherical graphite tank (250ml / can), in order to maintain the balan...
Embodiment 2
[0047] (1) Weigh 0.8499g NaNO 3 (analytical pure, Sinopharm Group Chemical Reagent Co., Ltd.) was dissolved in a beaker filled with 50ml deionized water, and 5.8320g Mg(OH) was added thereto 2 (super pure ≥ 99%, Sinopharm Chemical Reagent Co., Ltd.) and 0.6008g nanometer powder SiO 2 (analytical grade, Sinopharm Chemical Reagent Co., Ltd.), and stirred in an ultrasonic stirrer for 20 min in a water bath heating environment at 60°C.
[0048] (2) Place the above-mentioned beaker containing the mixed solution on a magnetic stirrer and heat it at a speed of 100 rpm until the solution evaporates to dryness; place the evaporated material in a vacuum drying oven and dry it at 80°C for 12 Hour.
[0049] (3) Use a planetary high-speed ball mill (QS92, Nanjing Nanda Instrument Factory) to dry-mill the dried material for 8 hours, using only one spherical graphite tank (250ml / can), in order to keep the running state balanced, the other three tanks The same amount of reference material ...
Embodiment 3
[0053] (1) Weigh 0.2896g LiNO 3 (analytical pure, Sinopharm Chemical Reagent Co., Ltd.) and 0.5864g KNO 3 (analytical pure, Sinopharm Group Chemical Reagent Co., Ltd.) was dissolved in a beaker filled with 50ml deionized water, and 5.8320g Mg(OH) was added thereto 2 (super pure ≥ 99%, Sinopharm Chemical Reagent Co., Ltd.) and 0.6008g nanometer powder SiO 2 (analytical grade, Sinopharm Chemical Reagent Co., Ltd.), and stirred in an ultrasonic stirrer for 20 min in a water bath heating environment at 60°C.
[0054] (2) Place the above-mentioned beaker containing the mixed solution on a magnetic stirrer and heat it at a speed of 100 rpm until the solution evaporates to dryness. The evaporated material was placed in a vacuum oven and dried at 80° C. for 12 hours.
[0055] (3) The dried material was dry-milled for 8 hours with a planetary high-speed ball mill (QS92, Nanjing Nanda Instrument Factory). Only use 1 spherical graphite tank (250ml / can), in order to maintain the balan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com