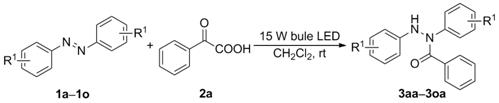
Synthesis method of N, N' -diaryl benzoyl hydrazine compound
A technology of aryl benzoyl hydrazide and synthesis method, which is applied in the field of synthesis of N,N'-diaryl benzoyl hydrazide compounds and can solve the hydroacylation reaction between azobenzene compounds and α-keto acid No problems such as reports have been reported, and the effects of high yield, simple and easy method, and wide application of substrates are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
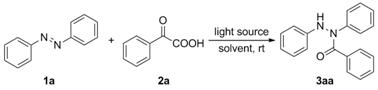
[0027] Example 1 A method for synthesizing N,N'-diarylbenzoic hydrazide compounds: the method refers to adding azobenzene 1a (36.4 mg, 0.2 mmol), benzene to dichloromethane (3.0 mL). Formic acid 2a (30.0 mg, 0.2 mmol, 1 equiv) was stirred at room temperature under 15 W blue LED irradiation to react for 24 h (complete reaction was monitored by TLC) to obtain a reaction mixture; the reaction mixture was distilled under reduced pressure to remove the solvent, After silica gel column chromatography, N,N'-diphenylbenzohydrazide (3aa) was obtained.
[0028]
[0029] White solid; yield: 55.9 mg (97%); m.p. 136‒138 ℃.
[0030] 1 H NMR (400 MHz, CDCl 3 ): δ = 7.56 (d, J = 7.6 Hz, 2H), 7.38 (t, J = 7.4Hz, 1H), 7.32–7.24 (m, 8H), 7.16 (t, J = 7.0 Hz, 1H), 7.09(s, 1H), 6.96–6.87(m, 3H).
[0031] 13 C NMR (150 MHz, CDCl 3 ): δ = 170.8, 147.1, 143.1, 134.8, 130.6, 129.2, 128.8, 128.5, 128.0, 126.1, 124.4, 121.2, 113.4.
[0032] HRMS (ESI): m / z [M+H] + calcd for C 19 H 1...
Embodiment 2
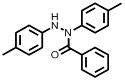
[0033] Example 2 A method for synthesizing N,N'-diarylbenzohydrazide compounds: the method refers to adding 4,4'-dimethylazobenzene 1b ( 43.0 mg, 0.2 mmol), benzoylformic acid 2a (30.0 mg, 0.2 mmol, 1 equiv), were stirred at room temperature under 15 W blue LED irradiation until the reaction was completed for 24 h (the reaction was monitored by TLC) to obtain a reaction mixture; The reaction mixture was distilled under reduced pressure to remove the solvent and subjected to silica gel column chromatography to obtain N,N'-bis(p-tolyl)benzohydrazide (3ba).
[0034]
[0035] White solid; Yield: 60.1 mg, (95%); m.p. 125–126 ℃.
[0036] 1 H NMR (600 MHz, CDCl 3 ): δ =7.53 (d, J= 7.8 Hz, 2H), 7.37–7.34 (m, 1H), 7.28–7.25 (m, 2H), 7.15 (d, J = 7.2 Hz, 2H), 7.04 (t, J = 7.5 Hz, 4H), 6.91(s, 1H), 6.86(d, J = 7.8 Hz, 2H), 2.28 (s, 3H), 2.26 (s, 3H).
[0037] 13 C NMR (150 MHz, CDCl 3 ): δ = 170.6, 144.7, 140.6, 136.0, 135.0, 130.7, 130.5, 129.8, 129.4, 128.6, 127.9, 124.6,...
Embodiment 3
[0039] Example 3 A method for synthesizing N,N'-diarylbenzoic hydrazide compounds: this method refers to adding 4,4'-di-n-butylazobenzene 1c to dichloromethane (3.0 mL). (58.9 mg, 0.2 mmol), benzoylformic acid 2a (30.0 mg, 0.2 mmol, 1 equiv), stirred at room temperature under 15 W blue LED irradiation until the reaction was completed for 24 h (the reaction was monitored by TLC) to obtain a reaction mixture ; After the reaction mixture was distilled under reduced pressure to remove the solvent and subjected to silica gel column chromatography, N,N'-bis(4-butylphenyl)benzohydrazide (3ca) was obtained.
[0040]
[0041] White solid; Yield: 68.1 mg (85%); m.p. 69‒70 ℃.
[0042] 1 H NMR (600 MHz, CDCl 3 ): δ = 7.52 (d, J = 7.8 Hz, 2H), 7.34 (t, J = 7.5Hz, 1H), 7.25 (t, J = 7.5 Hz, 2H), 7.17 (d, J = 7.8 Hz, 2H), 7.05 (t, J = 7.2Hz, 4H), 6.91 (s, 1H), 6.88 (d, J = 7.8Hz, 2H), 2.54 (q, J = 8.4 Hz, 4H), 1.57–1.53 (m, 4H), 1.36–1.30 (m, 4H), 0.92 (q, J =8.4 Hz, 6H). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com