Method for constructing full-retina in-vitro culture model suffering from diabetes retinopathy
A technology of diabetic retina and construction method, which is applied in the field of animal disease models, can solve the problems of ganglion cell apoptosis and short retinal survival time, and achieve good reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0037] Embodiment 1 Construction of DR model
[0038] 1 Culture medium preparation: the basal medium used for retinal in vitro culture does not contain serum, and is mixed with Neurobasal A, DMEM / F12 culture solution and N2 / B27 supplement at a volume ratio of 50:40:2, and added with a final concentration of 5 mg / L Insulin, 7.5mM D-glucose, 10% (0.1g / ml) BSA and 0.1mM cpt-cAMP were prepared.
[0039] 2 retinal dissection:
[0040] The entire operation must be performed in a sterile environment with all instruments sterilized.
[0041]Ways of killing SD rats at P8: Excessive chloral hydrate or neck dislocation; way of killing embryonic pigs: anesthetized with pentobarbital sodium (intravenous injection about 30 mg / kg) according to body weight, abdominal aorta or femoral artery Euthanasia by exsanguination.
[0042] After the animals were sacrificed, the retinal peeling was carried out according to the following steps: a. Carefully cut off the skin covering the eyeball surface...
Embodiment 2
[0044] Comparison of embodiment 2 with hypertonic control group and normal control group
[0045] 1. Method
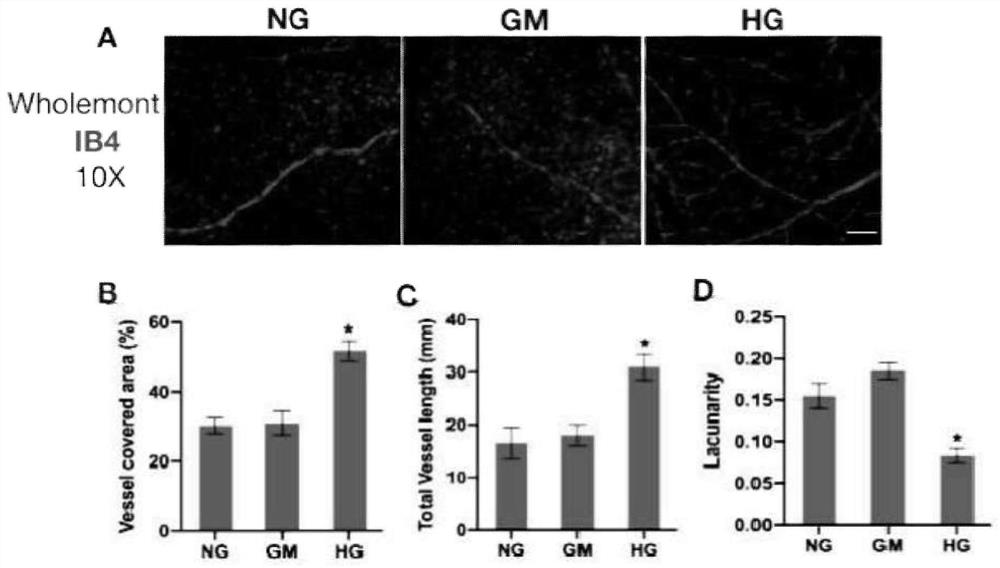
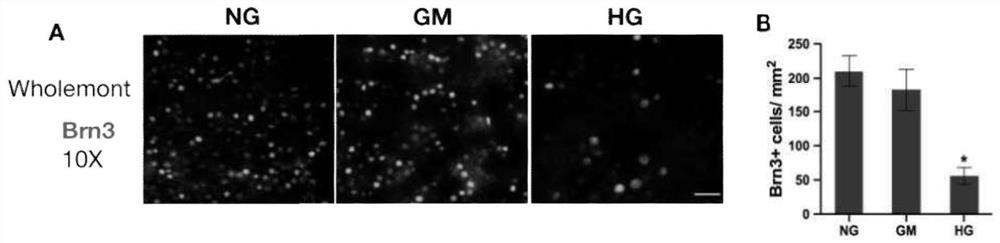
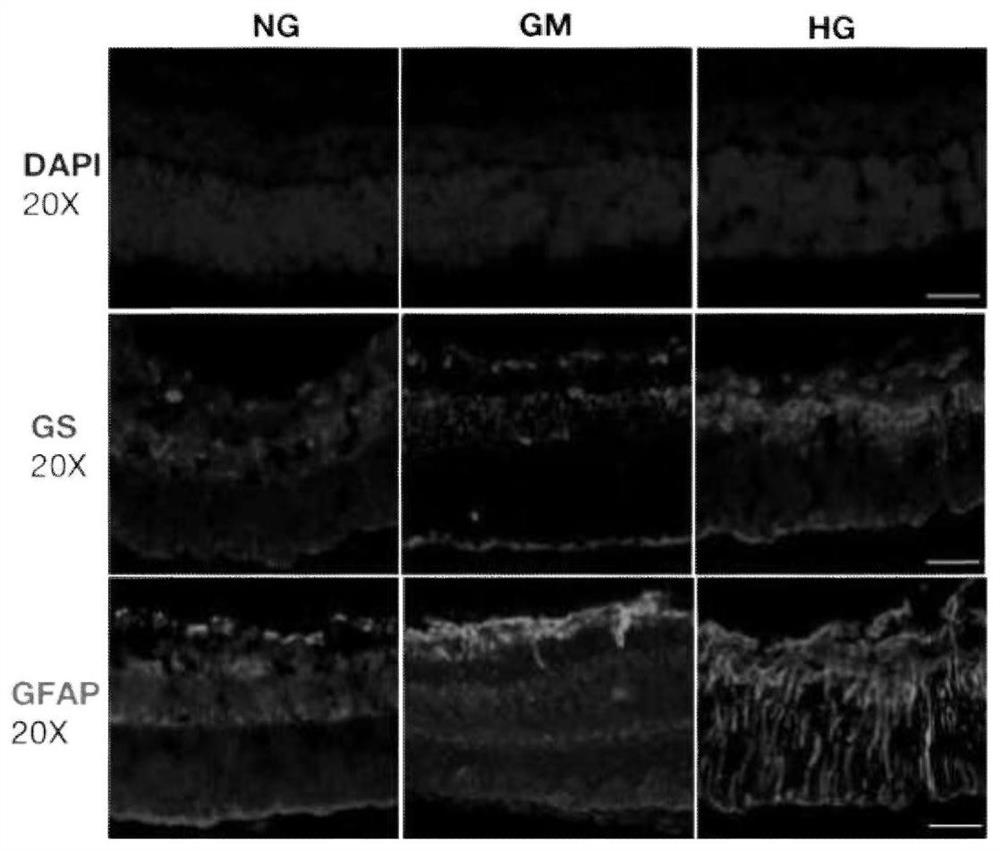
[0046] The retinas of SD rats 8 days after birth (P8) and 90-day embryonic pigs were peeled off according to the method of Example 1, divided into 3 groups, and cultured in three kinds of medium respectively, the temperature of the incubator was set at 37 ° C, CO 2 The content is 5%, cultured for 7 days, and the medium is changed every 2-3 days.
[0047] The medium was prepared as follows:
[0048] High glucose group (HG group): 27.5mmol / L (mM) D-glucose was added to the basal medium (plus the original D-glucose in the basal medium, the final concentration of D-glucose was 35mmol / L);
[0049] Hypertonic control group (GM group): 27.5mmol / L (mM) mannitol was added to the basal medium;
[0050] Normal control group (NG group): basal medium.
[0051] The basal medium is the same as the basal medium in Example 1.
[0052] Immunofluorescent staining was performed on fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com