Method for detecting rasagiline and enantiomer thereof
A technology of enantiomers and ultraviolet detectors, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as the inability to separate and detect rasagiline mesylate, and achieve good separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
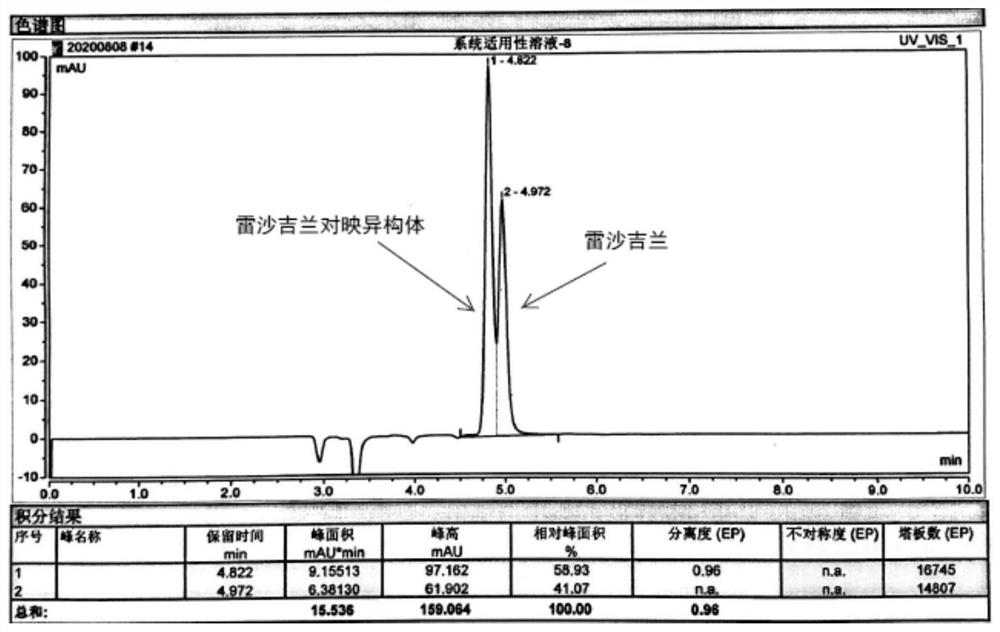
[0026] Use Agilent 1260 high performance liquid chromatograph, ultraviolet detector, chromatographic column (CHIRALCEL OD) with 3,5-dimethyl benzoate derivatized cellulose bonded silica gel as filler, detection wavelength is 272nm, column temperature is 30°C, the mobile phase is n-hexane-isopropanol-diethylamine (90:10:0, the volume ratio, the same below), the flow rate is 1.0ml / min, and the injection volume is 20μl. Get each 10mg of rasagiline mesylate reference substance and rasagiline enantiomer reference substance, weigh accurately, place in 100ml measuring bottle, add n-hexane-isopropanol (90:10, be volume Ratio, the same below) dissolved and diluted to the mark, shaken up, as the test solution. The test solution is injected into the chromatographic column, and the results are shown in figure 1 .
[0027] It can be seen from Figure 1 that, figure 1 The first peak from left to right in the middle is the enantiomer of rasagiline, and the second peak is rasagiline. The tw...
Embodiment 2
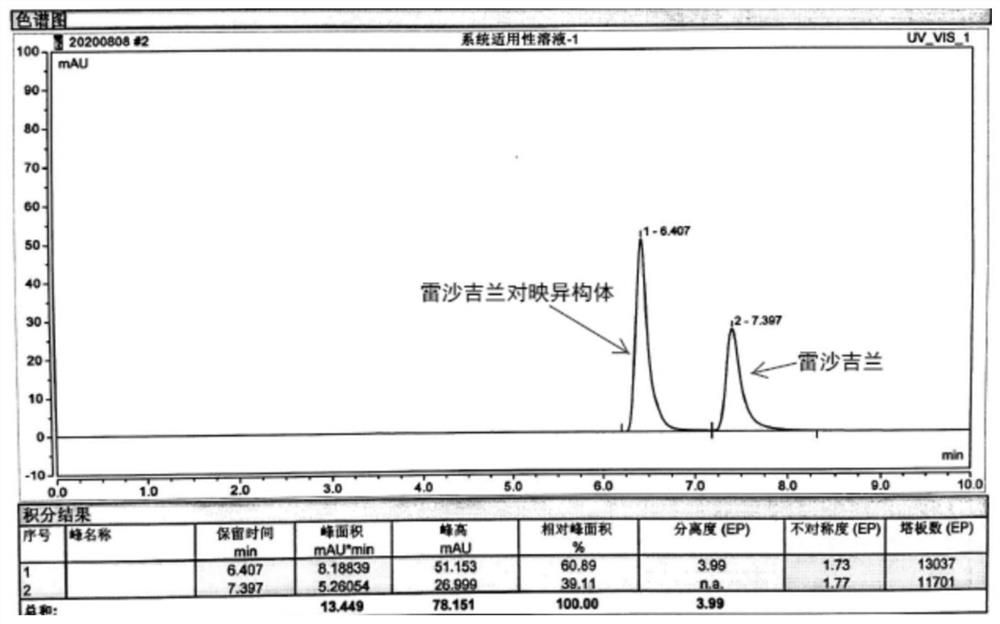
[0029] Use Agilent 1260 high performance liquid chromatography, UV detector, chromatographic column (CHIRALCEL AD) with 3,5-dimethylbenzoate derivatized amylose-bonded silica gel as filler, detection wavelength is 272nm, column temperature The temperature is 30°C, the mobile phase is n-hexane-isopropanol-diethylamine (90:10:0), the flow rate is 1.0ml / min, and the injection volume is 20μl. Get each 10mg of rasagiline mesylate reference substance and rasagiline enantiomer reference substance, accurately weighed, place in 100ml measuring bottle, add n-hexane-isopropanol (90:10) to dissolve and Dilute to the mark and shake well as the reference solution. Inject the reference substance solution into the chromatographic column, see the results figure 2 .
[0030] Depend on figure 2 It can be seen that the two peaks have been clearly separated. Although the separation degree of the two peaks is greater than 3.0, the peak tails and the asymmetry is greater than 1.2.
Embodiment 3
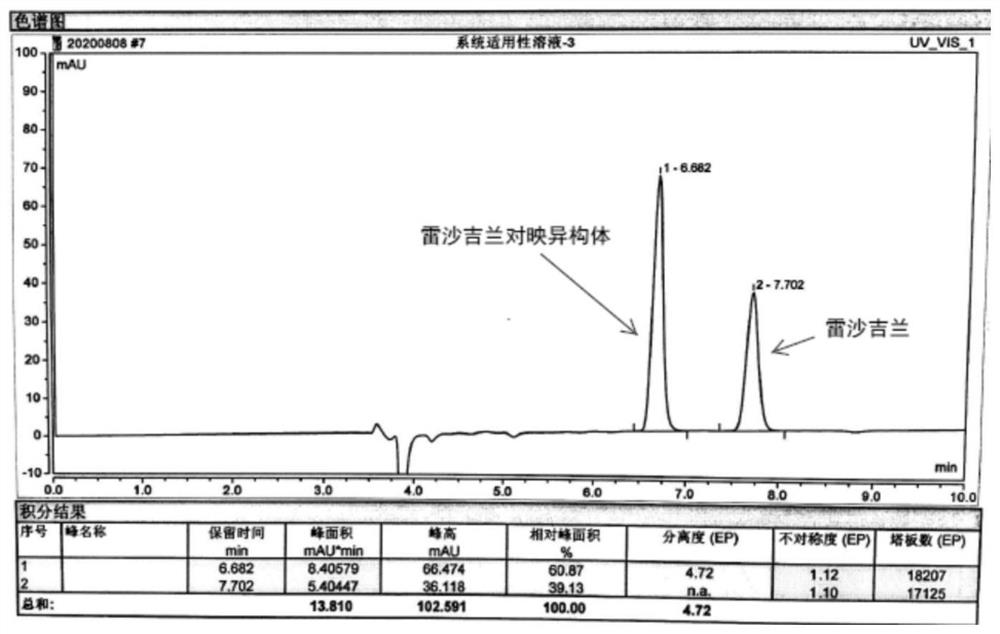
[0032] Use Agilent 1260 high performance liquid chromatograph, ultraviolet detector, the chromatographic column (CHIRALCELAD) with 3,5-dimethyl benzoate derivatized amylose-bonded silica gel as filler, the detection wavelength is 272nm, and the column temperature is 30°C, the mobile phase is n-hexane-isopropanol-diethylamine (90:10:0.1), the flow rate is 1.0ml / min, and the injection volume is 20μl. Get each 10mg of rasagiline mesylate reference substance and rasagiline enantiomer reference substance, accurately weighed, place in 100ml measuring bottle, add n-hexane-isopropanol-diethylamine (90: 10:0.1) was dissolved and diluted to the mark, shaken well, as the reference substance solution. Inject the reference substance solution into the chromatographic column, see the results image 3 .
[0033] Depend on image 3 It can be seen that after adding 0.1% diethylamine in the mobile phase, the peak shape is obviously improved, and the separation degree of the two peaks is great...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com