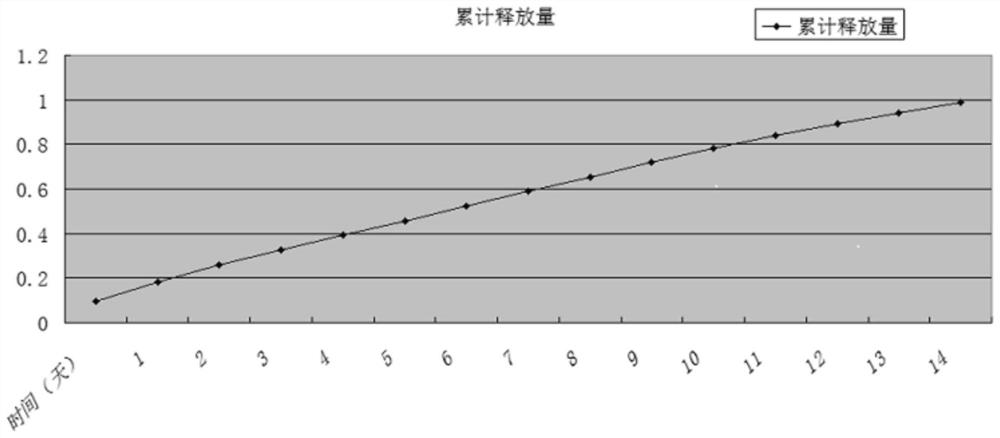
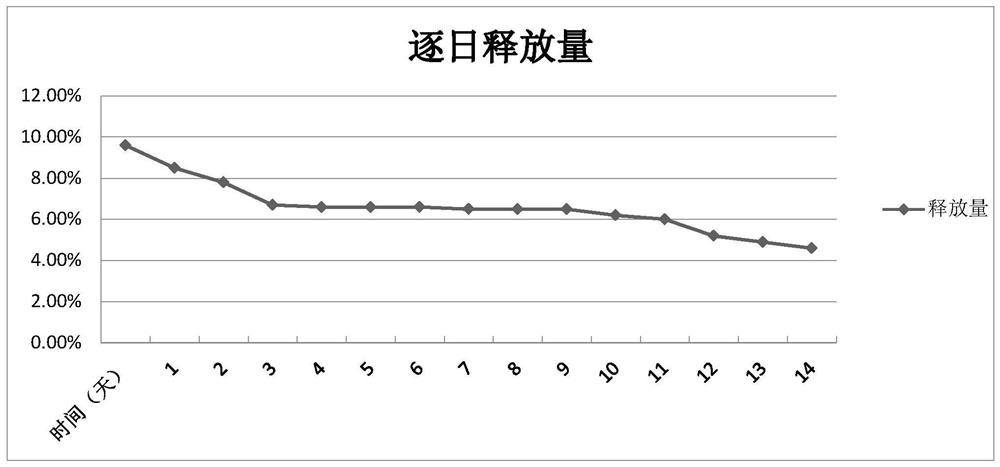
Drug delivery system of chitosan carrier and preparation method of drug delivery system
A drug-carrying system, chitosan technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of strong side effects, toxicity, etc., to achieve strong reproducibility, production The equipment is mature and the effect of improving treatment benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A drug-carrying system of chitosan carrier, comprising chitosan drug carrier and loaded cisplatin;
[0029] The acetylation degree of the chitosan drug carrier is 70;
[0030] The chitosan drug carrier is preferably chitosan polyethylene glycol;
[0031] Chitosan in the chitosan polyethylene glycol: the mass ratio of polyethylene glycol is preferably 75:25;
[0032] The loaded cisplatin is preferably a cisplatin solid preparation;
[0033] The mass ratio of the loaded cisplatin: chitosan polyethylene glycol is preferably 25:75.
[0034] A preparation method of a drug-carrying system of a chitosan carrier, comprising the following steps:
[0035] (1) Weigh 15 g of chitosan, 5 g of polyethylene glycol, and 6.6 g of cisplatin; place chitosan, polyethylene glycol, and cisplatin in a sealed operating table successively into a sealed mixer and stir for 5 to 10 minutes;
[0036] (2) Put the mixed material into the hot-melt extruder through the feeding port;
[0037] (3) S...
Embodiment 2
[0041] A drug-carrying system of chitosan carrier, comprising chitosan drug carrier and loaded cisplatin;
[0042] The acetylation degree of the chitosan drug carrier is 70;
[0043] The chitosan drug carrier is preferably chitosan polyethylene glycol;
[0044] Chitosan in the chitosan polyethylene glycol: the mass ratio of polyethylene glycol is preferably 50:25;
[0045] The loaded cisplatin is preferably a cisplatin solid preparation;
[0046] The mass ratio of the loaded cisplatin: chitosan polyethylene glycol is preferably 10:50.
[0047] A preparation method of a drug-carrying system of a chitosan carrier, comprising the following steps:
[0048] (1) Weigh 10 g of chitosan, 5 g of polyethylene glycol, and 3 g of cisplatin; place chitosan, polyethylene glycol, and cisplatin in a sealed operating table successively into a sealed mixer and stir for 5 to 10 minutes;
[0049] (2) Put the mixed material into the hot-melt extruder through the feeding port;
[0050] (3) Set...
Embodiment 3
[0055] A drug-carrying system of chitosan carrier, comprising chitosan drug carrier and loaded cisplatin;
[0056] The acetylation degree of the chitosan drug carrier is 70;
[0057] The chitosan drug carrier is preferably chitosan polyethylene glycol;
[0058] Chitosan in the chitosan polyethylene glycol: the mass ratio of polyethylene glycol is preferably 75:50;
[0059] The loaded cisplatin is preferably a cisplatin solid preparation;
[0060]The mass ratio of the loaded cisplatin: chitosan polyethylene glycol is preferably 50:90.
[0061] A preparation method of a drug-carrying system of a chitosan carrier, comprising the following steps:
[0062] (1) Weigh 15 g of chitosan, 10 g of polyethylene glycol, and 13.8 g of cisplatin; place chitosan, polyethylene glycol, and cisplatin in a sealed operating table in turn into a sealed mixer and stir for 5 to 10 minutes;
[0063] (2) Put the mixed material into the hot-melt extruder through the feeding port;
[0064] (3) Set t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com