Full racemic phytol composition, and preparation method and application thereof
A technology of phytoalcohol and composition, which is applied in the application field of the whole racemic phytoalcohol composition and the preparation of vitamin K1, can solve the problems of reducing, not providing, and difficult to control the content of Z-isomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
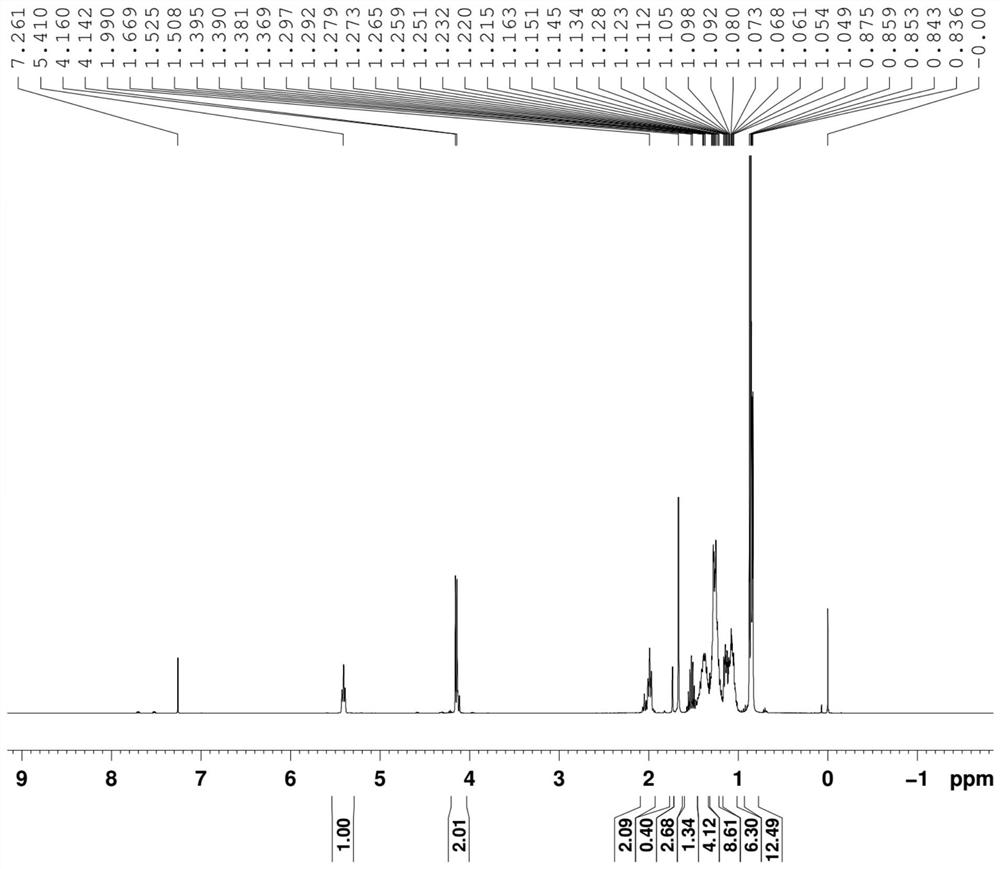
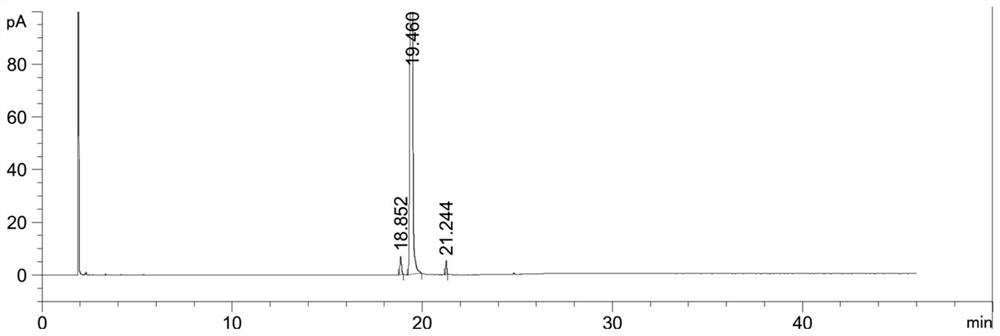
[0083] Example 1 Preparation of the all-racemic phytoalcohol composition (formula VII) with high E-type configuration content
[0084] (1), the preparation of the composition shown in formula V-a
[0085]
[0086] Dissolve 5 kg of isophytic alcohol (formula III) in 18 kg of methyl tert-butyl ether, stir and cool to below -10°C, and add dropwise 2.28 kg of phosphorus tribromide and methyl tert-butyl ether under temperature control below 0°C 2kg of the solution prepared; after dropping, continue to control the temperature and react below 0°C; after TLC monitors the reaction, add 1 kg of water dropwise under the temperature control below 0°C; 5-6. Dry over anhydrous sodium sulfate, filter, and concentrate to obtain the composition represented by formula V-a, which is directly used in the next reaction.
[0087] (2), the preparation of the composition shown in formula VIII-a
[0088]
[0089] Add the composition represented by the formula V-a obtained above, 268 g of tetrabu...
Embodiment 2
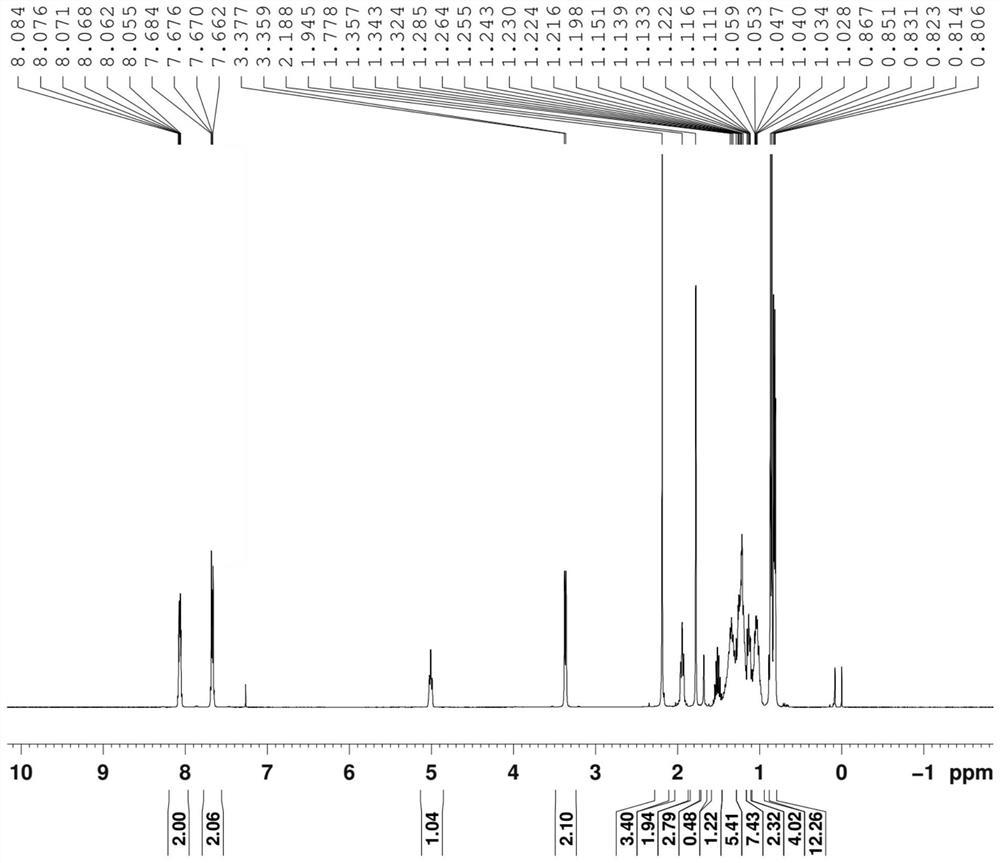
[0095] Example 2 Preparation of the all-racemic phytoalcohol composition (formula VII) with high E-type configuration content
[0096] (1), the preparation of the composition shown in formula V-b
[0097]
[0098] Mix 0.5kg of isophytic alcohol (formula III), 3.5kg of concentrated hydrochloric acid and 20g of tetramethylammonium chloride, and stir the reaction at 10-20°C under temperature control; after the reaction is monitored by TLC, add n-heptane for extraction; the obtained organic The phase was washed with water to pH=5-6, dried over anhydrous sodium sulfate, filtered and concentrated to obtain the composition represented by formula V-b, which was directly used in the next reaction.
[0099] (2), the preparation of the composition shown in formula VIII-b
[0100]
[0101] Add the composition shown in the formula V-b obtained above, 30 g of tetramethylammonium chloride and 344 g of sodium formate into 5 kg of acetonitrile, and stir the reaction at 50-60 ° C; TLC mo...
Embodiment 3
[0107] Example 3 Preparation of the all-racemic phytoalcohol composition (formula VII) with high E-type configuration content
[0108] (1), the preparation of the composition shown in formula V-a
[0109]
[0110] Dissolve 5 kg of isophytic alcohol (formula III) in 18 kg of methyl tert-butyl ether, stir and cool to below -10°C, and add 2.28 kg of phosphorus tribromide and methyl tert-butyl ether dropwise under temperature control below 0°C 2kg of the solution; after dropping, continue to control the temperature below 0°C to react; after the reaction is monitored by TLC, add 1 kg of water dropwise at the temperature below 0°C; The aqueous solution was washed until the pH of the aqueous phase = 5-6, and the obtained organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain the composition represented by formula V-a, which was directly used in the next reaction.
[0111] (2), the preparation of the composition shown in formula VIII-a
[0112...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com