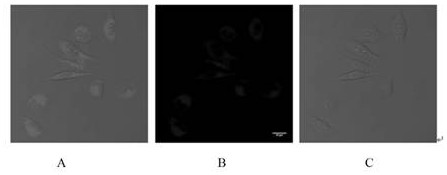
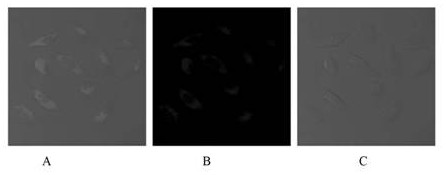
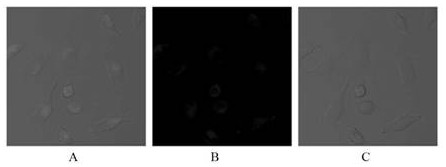
Preparation method and application of tetraphenyl ethylene derivative containing imidazole ring structure
A technology of tetraphenylethylene and imidazole rings, applied in the preparation and application in cell staining, the synthesis and application of TPE imidazole ring fluorescent dyes, can solve the low sensitivity of fluorescent sensing systems and restrict the application and development of fluorescent dyes and other problems, to achieve the effects of simple post-treatment, simple synthesis method, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of compound 1
[0036] Weigh 4-(1,2,2-triphenylvinyl)benzaldehyde (53.8mg, 0.15mmol), ammonium acetate (57.9mg, 0.75mmol) and 1,10-phenanthroline-5,6-di The ketone (37.9mg, 0.18mmol) was dissolved in 5ml of acetic acid and refluxed at 110°C for 3.5h. After the reaction was completed, the reaction solution was poured into 20 mL of water, and an orange solid was precipitated. After filtering, washing with water and drying, an orange solid was obtained, namely dye 1, with a yield of 58.9%.
Embodiment 2
[0037] Embodiment 2: the synthesis of compound 2
[0038] Weigh 4-(1,2,2-triphenylvinyl)benzaldehyde (54.1mg, 0.15mmol), ammonium acetate (57.8mg, 0.75mmol) and 9,10-phenanthrenequinone (37.5mg, 0.18mmol) Dissolved in 5ml of acetic acid, reflux reaction at 110°C for 3.5h. After the reaction, the reaction solution was poured into 20 mL of water, and an orange solid was precipitated. After filtering, washing with water and drying, a light yellow solid was obtained, namely dye 2, with a yield of 78.4%.
Embodiment 3
[0039] Embodiment 3: the synthesis of compound 3
[0040] Weigh 4-(1,2,2-triphenylvinyl)benzaldehyde (72mg, 0.2mmol), ammonium acetate (77mg, 1mmol) and benzil (63mg, 0.3mmol) and dissolve in 5ml acetic acid, in Reflux reaction at 110°C for 3.5h. After the reaction, the reaction solution was poured into 20 mL of water, and a yellow solid was precipitated. After filtering, washing with water, and drying, a white solid was obtained, namely dye 3, with a yield of 50.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com