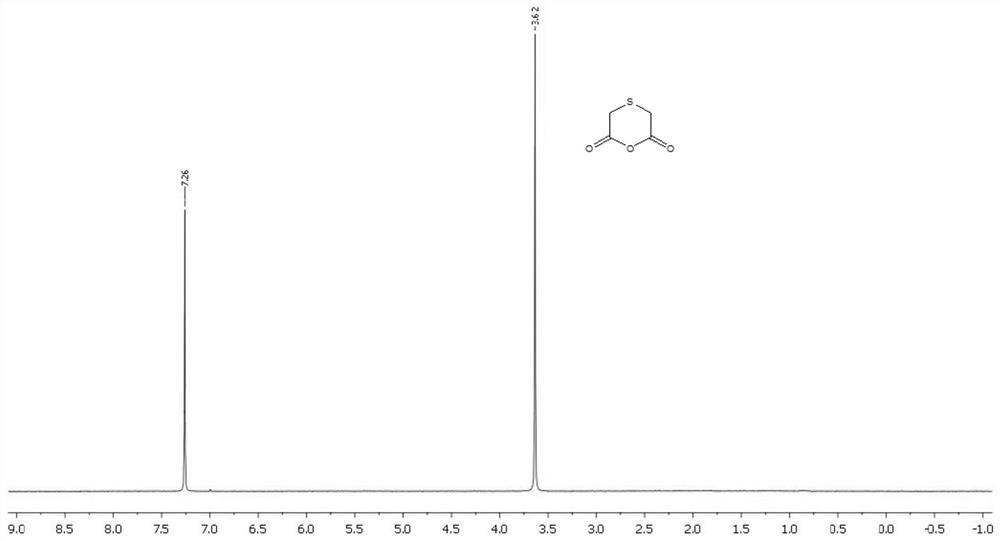
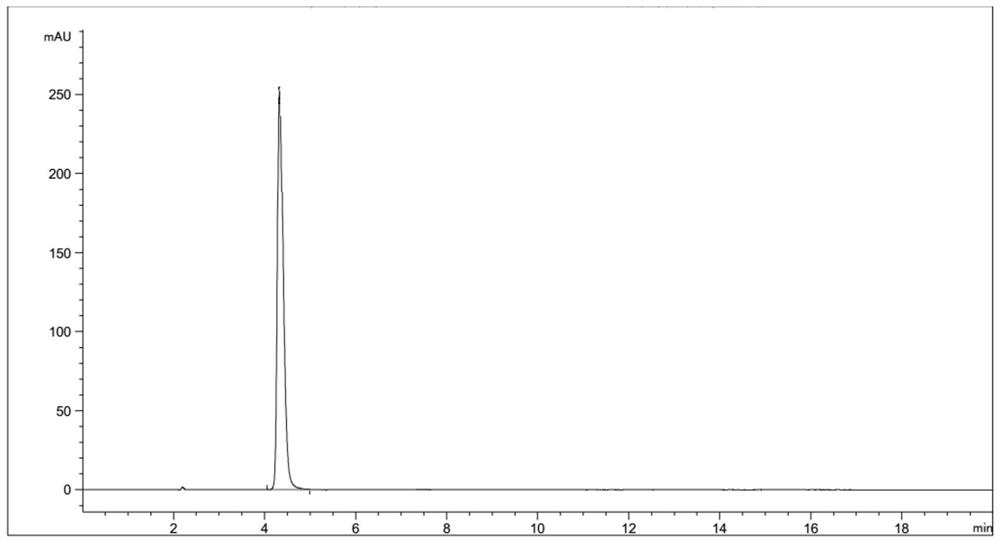
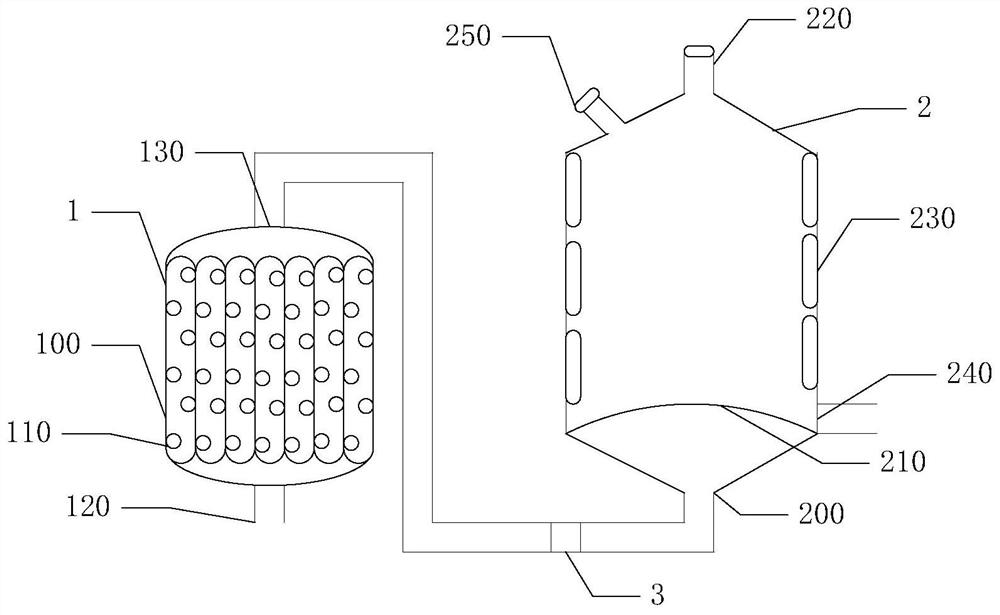
Preparation method of thioglycolic anhydride
A technology of thioglycolic anhydride and thiodiacetic acid, which is applied in the field of preparation of erdosteine intermediates, can solve the problems of low quality, long reaction time, and low reaction yield in the synthesis of erdosteine, and achieve Improved reaction efficiency, shortened reaction time, and improved contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation method of thioglycolic anhydride, comprising the following steps:
[0040] 1) Vaporization of trifluoroacetic anhydride: vaporize trifluoroacetic anhydride in a vaporization device for use;
[0041] 2) Synthesis of thioglycolic anhydride: After preheating the internal temperature of the suspended gas-solid reaction device to 55° C., the vaporized trifluoroacetic anhydride is introduced from the air inlet provided at the bottom of the suspended gas-solid reaction device. In the suspended gas-solid reaction device, the gas in the suspended gas-solid reaction device is detected by a gas detector. When the gas detector shows that there is no air in the suspended gas-solid reaction device, continue to feed the vaporized trifluoroethylene Acid anhydride to the flow rate that can make the powder thiodiacetic acid can be stably suspended in the high-temperature trifluoroacetic acid airflow (this numerical value is set in advance before the start of the reaction, a...
Embodiment 2
[0044] A preparation method of thioglycolic anhydride, comprising the following steps:
[0045] 1) Vaporization of trifluoroacetic anhydride: vaporize trifluoroacetic anhydride in a vaporization device for use;
[0046] 2) Synthesis of thioglycolic anhydride: after preheating the internal temperature of the suspended gas-solid reaction device to 45°C, the vaporized trifluoroacetic anhydride is introduced from the air inlet provided at the bottom of the suspended gas-solid reaction device In the suspended gas-solid reaction device, the gas in the suspended gas-solid reaction device is detected by a gas detector. When the gas detector shows that there is no air in the suspended gas-solid reaction device, continue to feed the vaporized trifluoroethylene Acid anhydride to the flow rate that can make the powder thiodiacetic acid can be stably suspended in the high-temperature trifluoroacetic acid airflow (this numerical value is set in advance before the start of the reaction, and ...
Embodiment 3
[0049] A preparation method of thioglycolic anhydride, comprising the following steps:
[0050] 1) Vaporization of trifluoroacetic anhydride: vaporize trifluoroacetic anhydride in a vaporization device for use;
[0051] 2) Synthesis of thioglycolic anhydride: after preheating the internal temperature of the suspended gas-solid reaction device to 50°C, the vaporized trifluoroacetic anhydride is introduced into the In the suspended gas-solid reaction device, the gas in the suspended gas-solid reaction device is detected by a gas detector. When the gas detector shows that there is no air in the suspended gas-solid reaction device, continue to feed the vaporized trifluoroethylene Acid anhydride to the flow rate that can make the powder thiodiacetic acid can be stably suspended in the high-temperature trifluoroacetic acid airflow (this numerical value is set in advance before the start of the reaction, and is recorded by the gas flow detector and passed to the controller for storag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com