Tofacitinib citrate intermediate as well as preparation method and application thereof
A technology of tofacitinib and citric acid, which is applied in the field of tofacitinib citrate intermediates and its preparation, can solve the problems of only 65% total yield and cumbersome process, and reduce production cost and reactivity High, avoid the effect of incomplete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine dihydrochloride Hydrate Synthesis
[0048] Add 33.5 g (0.10 mol) of N-methyl-N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrole to the autoclave And [2,3-d]pyrimidin-4-amine, add 20ml of water and 400ml of ethanol, add 10g (0.10mol) of hydrochloric acid, 1.5g of palladium hydroxide on carbon, stir, replace the air in the reactor with nitrogen, and heat up to 50°C , pass hydrogen gas to 0.3MPa to react for 8h, vent, filter out palladium hydroxide carbon, add 320ml of acetone dropwise to the reaction solution at room temperature, a large amount of solid precipitates, filter with suction, and dry at 45°C for 3h to obtain 32.7g of compound 1, yield 97.3%.
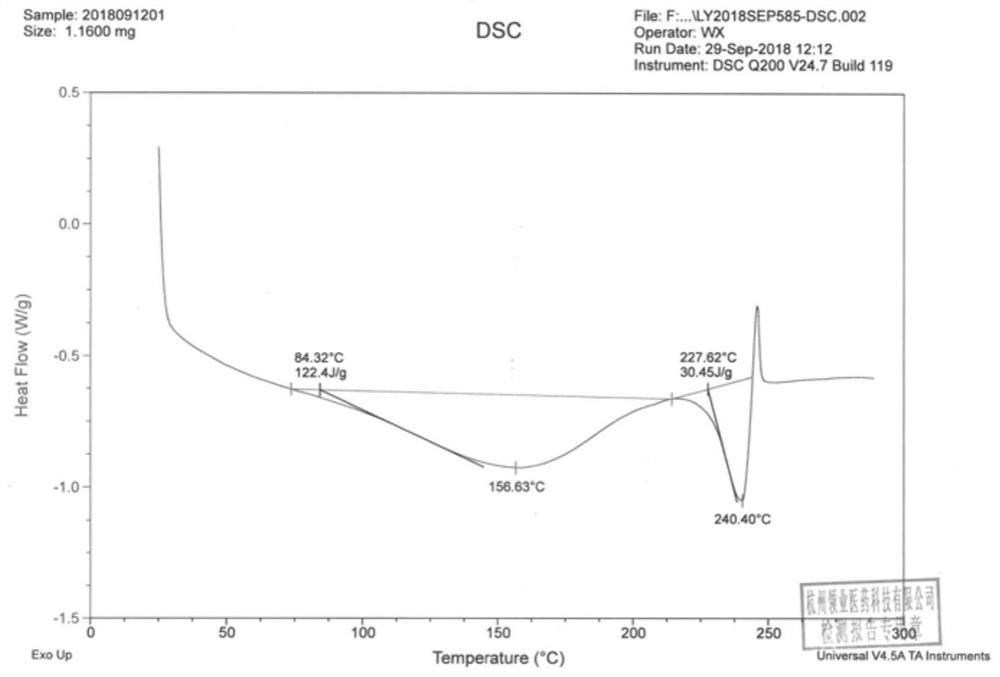
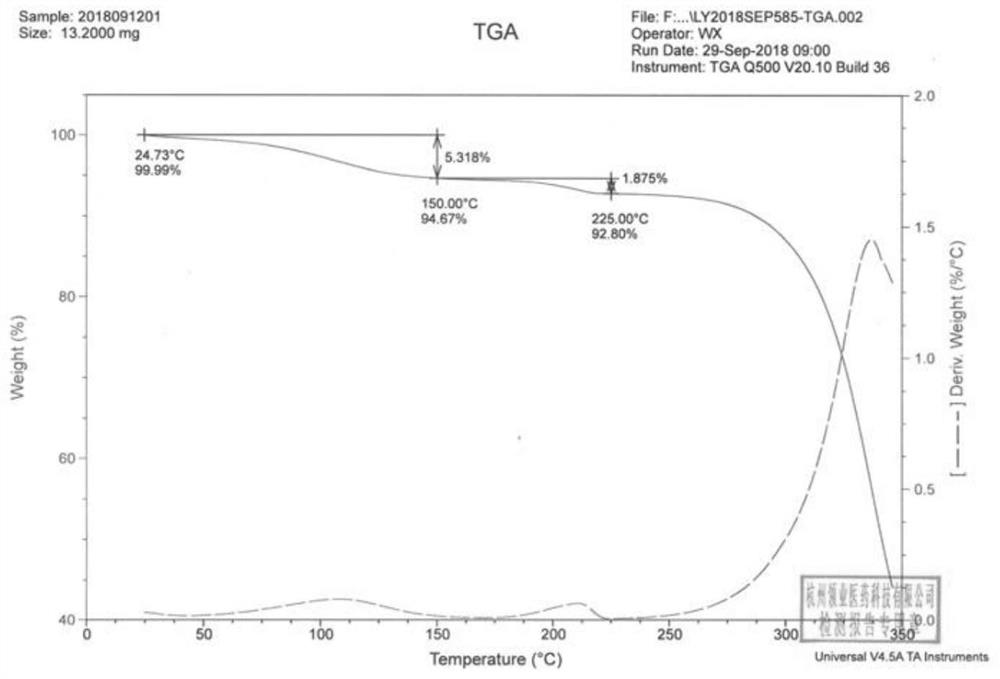
[0049] Compound 1 was analyzed: through DSC thermal analysis, compound 1 had a broad endothermic peak before 200°C, which was initially judged to be a desolvation endothermic peak; through TGA thermal analysis, compound 1 ha...
Embodiment 2
[0053] (1) N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine dihydrochloride Hydrate Synthesis
[0054] Add 33.5 g (0.10 mol) of N-methyl-N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrole to the autoclave And [2,3-d] pyrimidin-4-amine, add 30ml of water and 500ml of isopropanol, add 15g (0.15mol) of hydrochloric acid, 1.8g of palladium hydroxide carbon, stir, replace the air in the reactor with nitrogen, and heat up to At 60°C, pass hydrogen gas to 0.5MPa to react for 5h, vent, filter out palladium hydroxide carbon, add 280ml of tetrahydrofuran dropwise to the reaction solution at room temperature, a large amount of solid precipitates, filter with suction, and dry at 40°C for 5h to obtain 32.5g of compound 1. Yield 96.8%.
[0055] (2) Synthesis of Tofacitinib Citrate
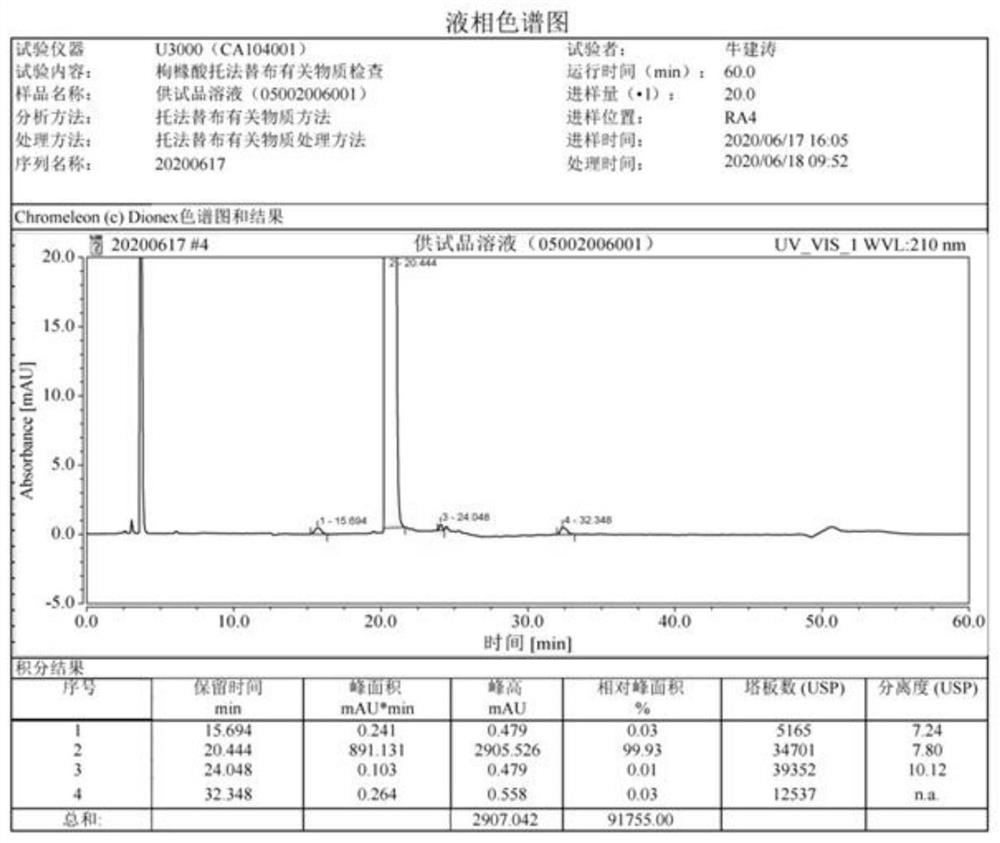
[0056] The preparation method was the same as the synthesis of tofacitinib citrate in Example 1 to obtain 39.1 g of tofacitinib citrate with a purity of 99.93%, no c...
Embodiment 3
[0058] (1) N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-D]pyrimidin-4-amine dihydrochloride Hydrate Synthesis
[0059] Add 33.5 g (0.10 mol) of N-methyl-N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrole to the autoclave And [2,3-d]pyrimidin-4-amine, add 25ml of water and 600ml of methanol, add 20g (0.20mol) of hydrochloric acid, 3.0g of palladium hydroxide on carbon, stir, replace the air in the reactor with nitrogen, and heat up to 60°C , pass hydrogen gas to 0.4MPa to react for 8h, vent, filter off palladium hydroxide carbon, add 200ml of acetonitrile dropwise to the reaction solution at room temperature, a large amount of solid precipitates, filter with suction, and dry at 50°C for 4h to obtain 32.6g of compound 1, yield 97.1%.
[0060] (2) Synthesis of Tofacitinib Citrate
[0061] The preparation method was the same as the synthesis of tofacitinib citrate in Example 1 to obtain 39.2 g of tofacitinib citrate with a purity of 99.93%, no compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com