Application of proteasome inhibitor in anti-cancer drugs
A proteasome inhibitor and anticancer drug technology, applied in the application field of proteasome inhibitors in anticancer drugs, can solve the problems such as the advent of immunotherapy programs, and achieve the effects of weakening the therapeutic effect, increasing infiltration, and improving the microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
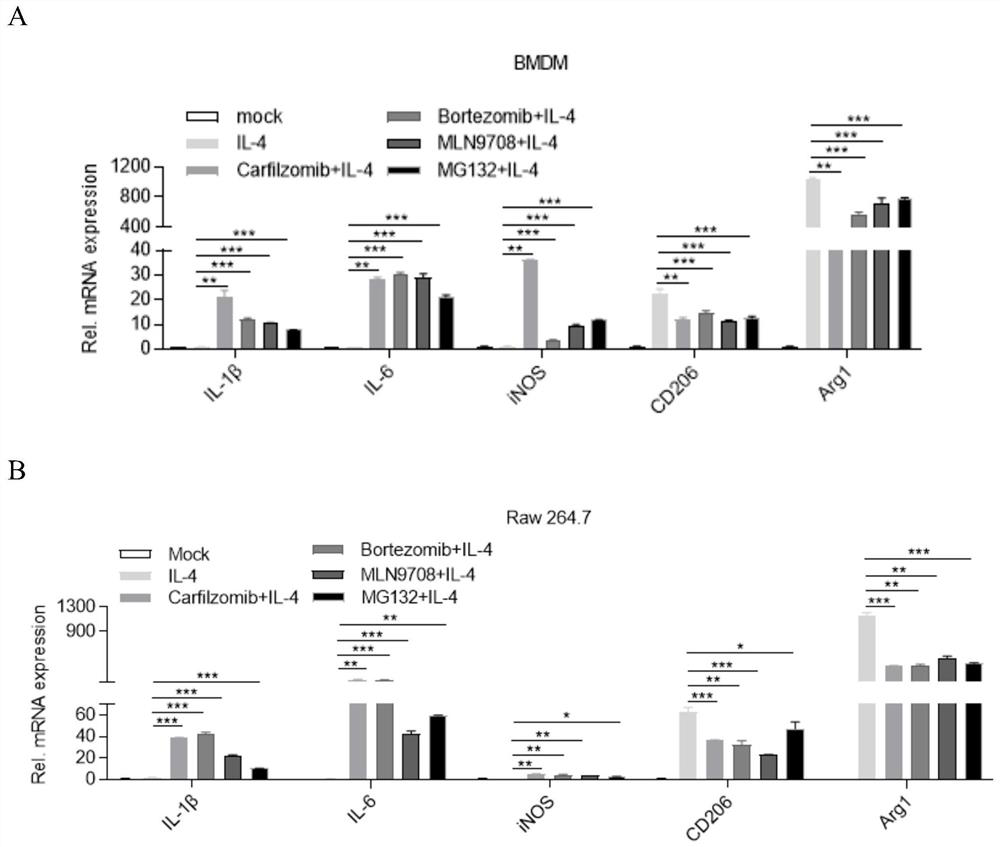
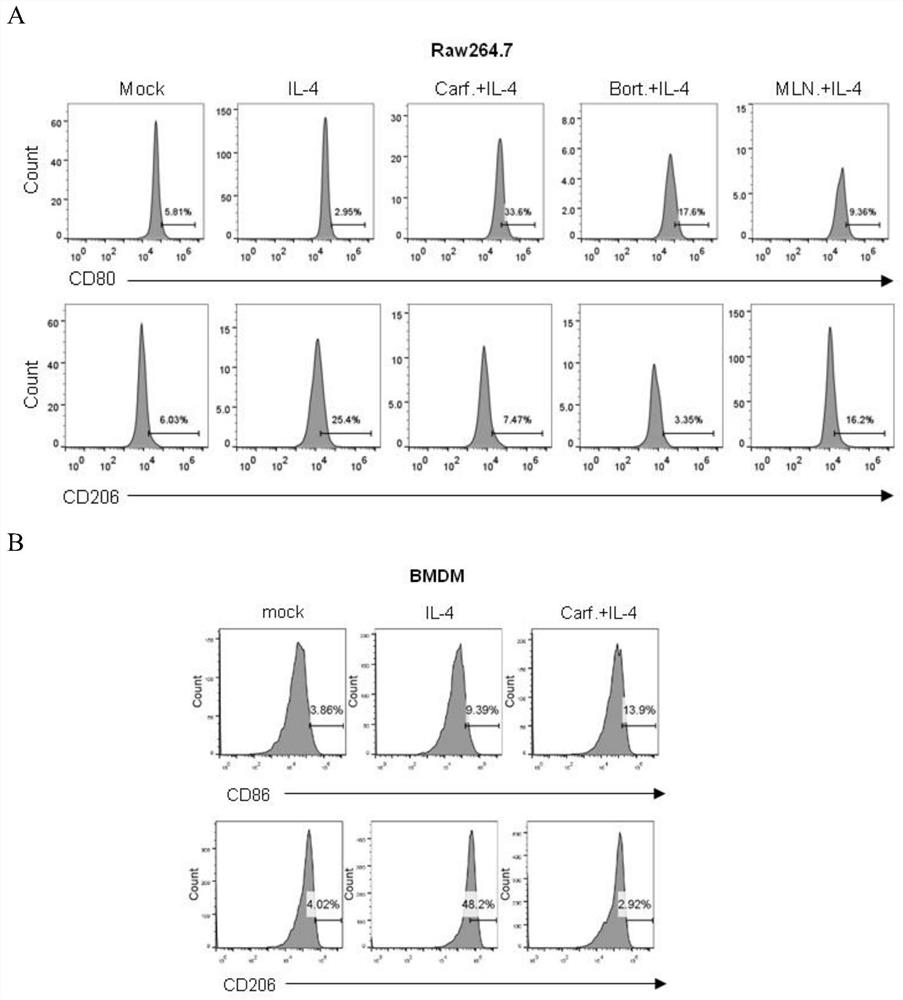
[0034] Proteasome inhibitors (Carfilzomib, Bortezomib, MLN9708, MG132) promote the expression of M1 macrophage markers while inhibiting the expression of M2 macrophage markers
[0035] 1. Experimental method
[0036] (1) Preparation of mouse bone marrow-differentiated macrophages: Take adult mice about 8 weeks old, kill them by cervical dislocation, soak them in 75% ethanol for 2 minutes, take them out, dissect and separate the mice For the legs, the coat and most of the muscles were removed, and then soaked in 75% ethanol for 10 minutes, then the leg bones were taken out, placed in PBS, and washed 2-3 times with PBS to remove residual ethanol. Use scissors and tweezers to separate the femur and tibia, and then peel off the articular cartilage at one end of the femur and tibia to expose the cross section, and cut the other end with scissors. Use a 10ml syringe to draw the PBS containing 1% serum and 1% penicillin-streptomycin mixture prepared in advance, wash the bone marrow ...
Embodiment 2
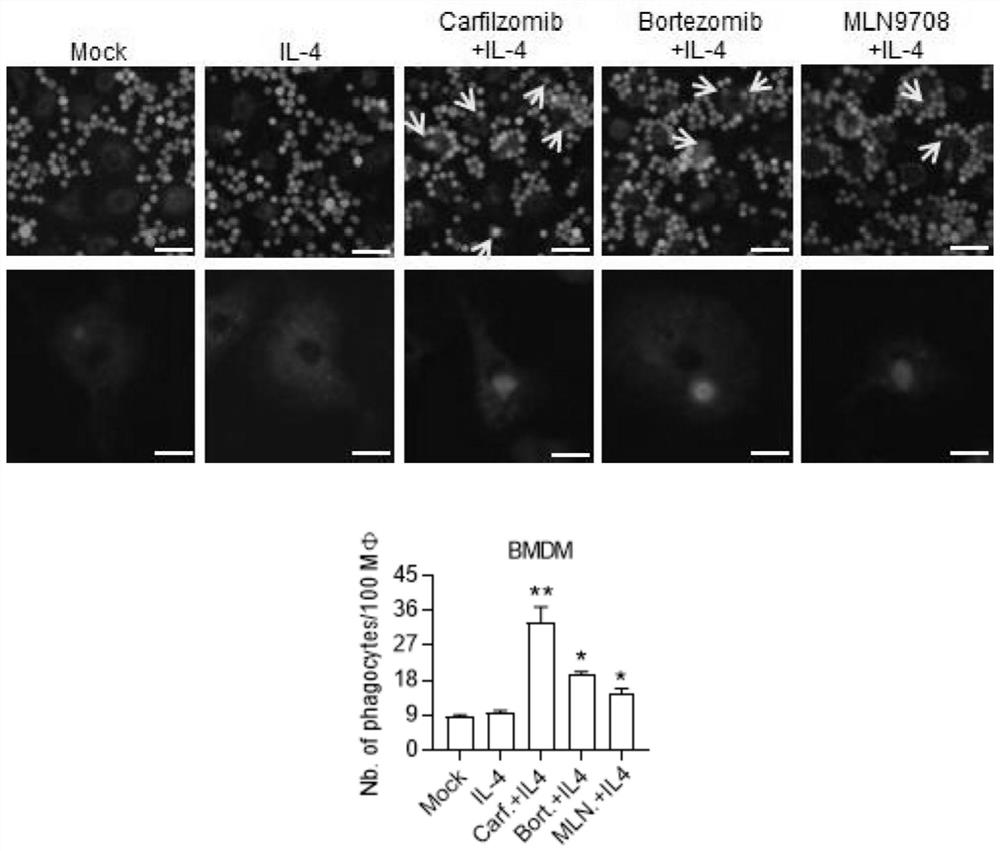
[0045] Proteasome inhibitor drugs Carfilzomib, Bortezomib and MLN9708 promote the phagocytosis of tumor cell L1210 by primary macrophage BMDM
[0046] 1. Experimental method:
[0047] (1) Induce BMDM differentiation: Inoculate 1×10 per well in a 12-well plate 5 Each BMDM was divided into five groups on the next day for different drug treatments, namely mock (blank group), IL-4 (20ng / ml), Carfilzomib (1μM), Bortezomib (1μM), MLN9708 (1μM) were pretreated for 1 hour and then Add IL-4 (20ng / ml) treatment group.
[0048] (2) Co-incubation of macrophages and tumor cells: After 12 hours of drug treatment, macrophages were replaced with serum-free medium, starved for 2-3 hours, and 4×10 5 Mouse leukemia cells (L1210-GFP) expressing GFP green fluorescent protein were incubated for 2 hours.
[0049] (3) Observation record: wash away the suspended L1210-GFP cells, observe the phagocytosis of target cells by macrophages under a fluorescence microscope, and take pictures for statistics...
Embodiment 3
[0053] Carfilzomib inhibits the growth of subcutaneous xenografts of mouse melanoma cell B16
[0054] 1. Experimental method
[0055] (1) The prepared mouse melanoma cell B16 was mixed with 100 μL of PBS and Matrigel (Matrigel) (1:1) containing 1×10^6 cells, and the tumor cell gel mixture was implanted into The flanks of 6-week-old BALB / c nude mice were injected with the mixture in a volume of 100 μl per implantation point.
[0056] (2) When the tumor volume reaches 80mm 3 When left or right, the mice were randomly divided into groups, one group was a normal saline control group (control group, tail vein injection, administered once every 2-3 days, 100 μl / time), and one group was a Carfilzomib treatment group (tail vein injection, Administration once every 2-3 days, each administration dose is 2mg / kg, 100μl / time), and the two groups of mice were administered synchronously.
[0057] (3) After 2 weeks of treatment, the mice were sacrificed, the tumors were dissected, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com