Preparation method of 6-deuterated dansyl chloride
A technology of dansyl chloride and deuteration, which is applied in the field of preparation of 6-deuterated dansyl chloride, can solve the problems of low yield and achieve the effects of less intermediate products, improved yield, high yield and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Prepare the reaction materials according to the following proportions:
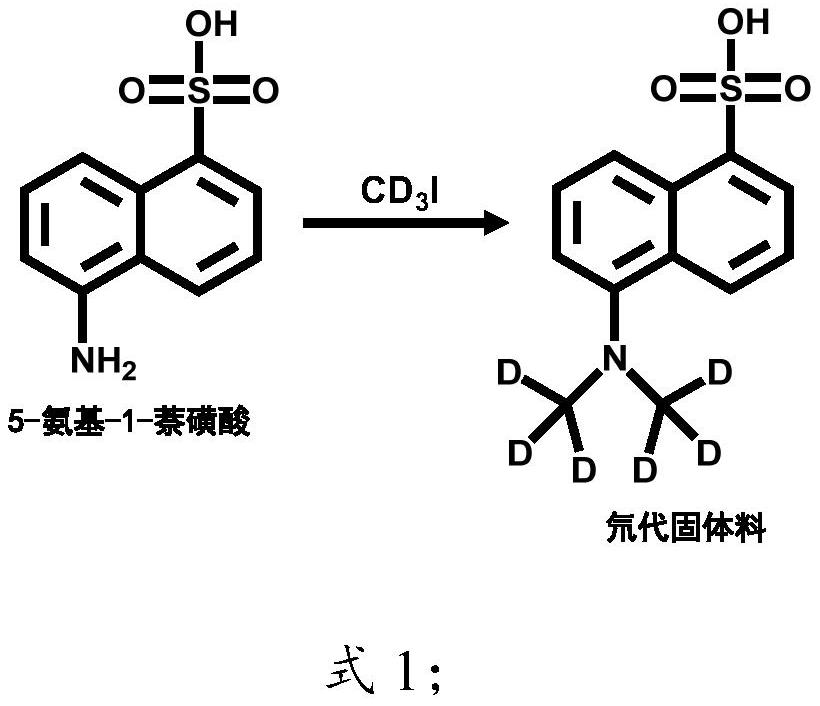
[0057] The mol ratio of sodium hydride and 5-amino-1-naphthalenesulfonic acid is 4.2:1;
[0058] The mol ratio of deuteroiodomethane and 5-amino-1-naphthalenesulfonic acid is 4.9:1;
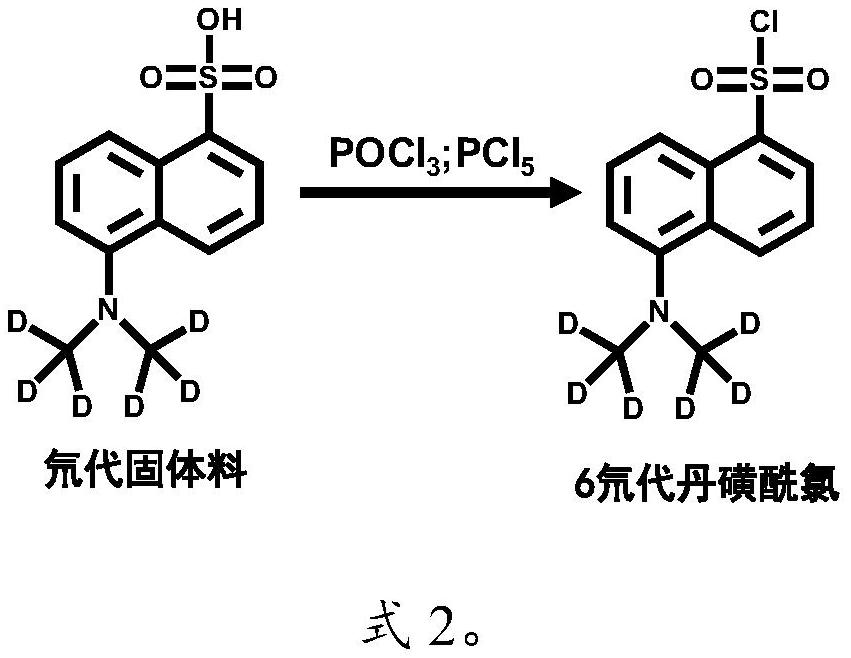
[0059] The molar ratio of phosphorus oxychloride, phosphorus pentachloride and deuterated solid material is 1.75:0.29:1, and the specific process and dosage are as follows:
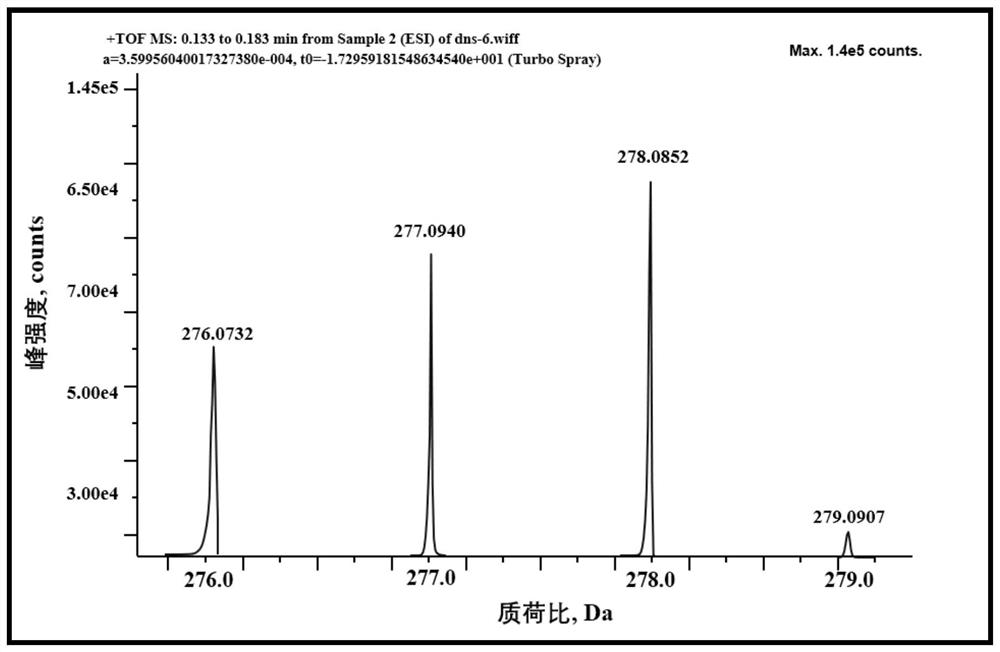
[0060] In the first step, suspend 448.4 mg (18.68 mmol) of sodium hydride solid in 1 mL of N,N-dimethylformamide, add 1 g (4.48 mmol) of 5-amino-1-naphthalenesulfonic acid, and cool to 0°C. Add 3.2g (22mmol) of CD 3 After 1, the temperature was raised to 35° C., and the reaction was carried out for 2 hours; water was added in an ice bath, and 6N HCl was added to adjust the pH of the solution to 3, and a solid was precipitated, filtered by suction, and dried at 120° C. for 2 hours to obtain a deuterated solid material;
[0061] In the second st...
Embodiment 2
[0064] Prepare 6 deuterated dansyl chlorides in the manner of Example 1, the difference is that the amount of raw materials and reaction conditions, wherein:
[0065] The mol ratio of sodium hydride and 5-amino-1-naphthalenesulfonic acid is 3.5:1;
[0066] The mol ratio of deuteroiodomethane and 5-amino-1-naphthalenesulfonic acid is 4.5:1;
[0067] The mol ratio of phosphorus oxychloride, phosphorus pentachloride and deuterated solid material is 2:0.3:1;
[0068] The temperature of the first substitution reaction was 37° C., and the reaction time was 1.5 h; the total yield was 73%, and the purity of the obtained target product was 73%.
Embodiment 3
[0070] Prepare 6 deuterated dansyl chlorides in the manner of Example 1, the difference is that the amount of raw materials and reaction conditions, wherein:
[0071] The mol ratio of sodium hydride and 5-amino-1-naphthalenesulfonic acid is 5:1;
[0072] The mol ratio of deuteroiodomethane and 5-amino-1-naphthalenesulfonic acid is 5.5:1;
[0073] The mol ratio of phosphorus oxychloride, phosphorus pentachloride and deuterated solid material is 1.8:0.35:1;
[0074] The temperature of the first substitution reaction is 30° C., the time is 2.5 h, the total yield is 82%, and the purity of the obtained target product is 80%.
[0075] As can be seen from the above examples, the method provided by the present invention can rapidly prepare 6-deuterated dansyl chloride, the reaction raw materials are easy to obtain, the preparation method is simple and easy to control, and the yield is high, reaching more than 75%; the overall reliability of the preparation method is high, and it is s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com