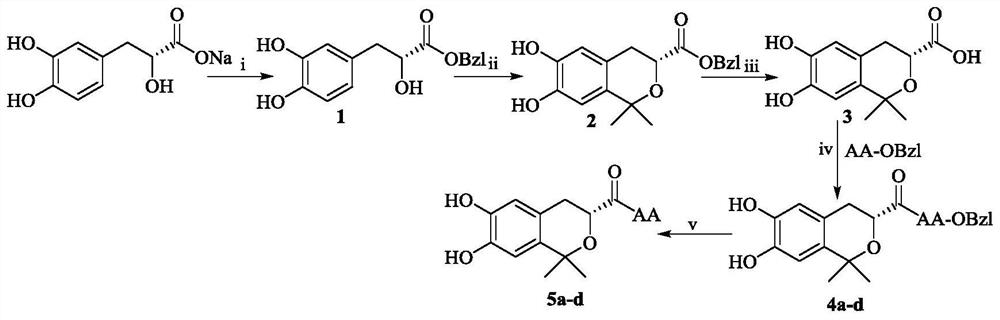
Dihydroxy dimethyl isochroman-3-formyl aromatic amino acid and preparation, thrombolytic activity and application thereof
A technology of dimethylisochroman and dihydroxyphenyl, which is used in thrombolytic activity, -6,7-dihydroxy-1,1-dimethylisochroman-3-formyl-AA, to prepare thrombolytic fields of application in medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of D(+)-β-(3,4-dihydroxyphenyl) benzyl lactate (1)
[0019] Slowly add 91.0 mL of thionyl chloride dropwise into 150 mL of benzyl alcohol stirred at 0°C. After dropping, stir at room temperature for 1h, add 55.0g (250mmol) danshensu sodium, stir at room temperature for 48h, and the reaction is complete. The reaction mixture was concentrated under reduced pressure, the residue was dissolved in 100 mL of ethyl acetate, washed with saturated NaCl aqueous solution (30 mL×3), washed with anhydrous NaCl 2 SO 4 Dry for 12 h, filter, and concentrate the filtrate under reduced pressure. The residue is purified by silica gel column chromatography to obtain 20.1 g (35%) of the title compound as a yellow oil. 1 HNMR (300M Hz, DMSO-d 6 ):δ / ppm=8.75(s,1H),8.67(s,1H),7.31(m,5H),6.61(s,1H),6.58(s,1H),6.42(dd,J 1 =1.8Hz,J 2 =2.1Hz,1H),5.55(d,J=6.0Hz,1H),5.12(s,2H),4.19(q,J 1 =6.9Hz,J 2 =6.0Hz,1H),2.73(qd,J 1 =8.1Hz,J 2 =5.4Hz, 2H); ESI-MS (m / e): 287 [M-H] ...
Embodiment 2
[0020] Example 2 Preparation of (R)-6,7-dihydroxy-1,1-dimethylisochroman-3-carboxylic acid benzyl ester (2)
[0021] 10.0 g (30.6 mmol) of benzyl D(+)-β-(3,4-dihydroxyphenyl)lactate (1) was dissolved in 104 mL of acetone. Slowly add 4.4mL of boron trifluoride diethyl ether dropwise under stirring at 0°C. After dropping, stirring at room temperature for 4h, compound 1 disappeared completely. The reaction solution was concentrated under reduced pressure, and the residue was dissolved in 100 mL of ethyl acetate. The resulting solution was washed with saturated NaCl aqueous solution (30mL×3), washed with anhydrous NaCl 2 SO 4 Dry for 12 h, filter, and concentrate the filtrate under reduced pressure. The residue is purified by silica gel column chromatography to obtain 11.4 g (80%) of the title compound as a yellow oil. 1 H NMR (300MHz, DMSO-d 6 ):δ / ppm=1.58(s,1H),1.54(s,1H),7.39(m,5H),6.52(s,1H),6.47(s,1H),1.93(s,1H),4.47( m,1H),2.71(m,2H),1.40(m,6H); ESI-MS(m / e):327[M-H] -...
Embodiment 3
[0022] Example 3 Preparation of (R)-6,7-dihydroxy-1,1-dimethylisochroman-3-carboxylic acid (3)
[0023] 11.4 g (34.8 mmol) of benzyl (R)-6,7-dihydroxy-1,1-dimethylisochroman-3-carboxylate (2) were dissolved in 60 mL of methanol. Then add 1.14g Pd / C, stir evenly, pass hydrogen, react at room temperature for 24h, compound 2 disappears completely. The reaction solution was filtered, and the filtrate was concentrated under reduced pressure to obtain 10.5 g (95%) of the title compound as a colorless solid. 1 H NMR (300MHz, DMSO-d 6 ):δ / ppm=12.60(s,1H),8.82(d,J=6.9Hz,2H),8.66(d,J=5.4Hz,2H),6.52(s,1H),6.46(s,1H) ,4.34(m,1H),2.68(d,J=7.2Hz,2H),1.37(d,J=3.8Hz,6H); ESI-MS(m / e):237[M-H] - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Tube chief | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com