PH responsive thrombolytic drug targeting nanogel, synthesis method and application thereof
A thrombolytic drug and nanogel technology, applied in the field of biomedicine, can solve the problems of reducing the biological activity of the drug, unstable immunogenicity, uneven drug-antibody coupling agent, etc., to prolong the time of hydrolysis and blood circulation. The effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
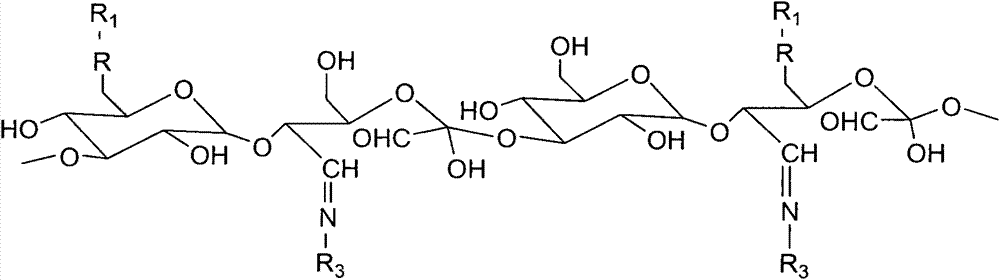
[0027] (1) Synthesis of oxidized dextran
[0028] Dissolve 1g of dextran (Mn 40000) in 30mL of water, add 0.956g of sodium periodate (dissolved in 20mL of water), and react in the dark for 24 hours at room temperature, then add 0.411g of glycerol, continue to stir for 15min, and mix the reaction The solution was transferred to a dialysis bag and dialyzed in deionized water for 48 h, changing the water every four hours. The dialyzed sample was freeze-dried to obtain the product as a white powder. By changing the molar ratio of dextran and sodium periodate, oxidized dextran with different aldehyde content can be prepared.
[0029] (2) Synthesis of oxidized dextran-RGD
[0030] Add 0.0382g RGD, 0.5g oxidized dextran (oxidation degree 0.5) and 0.1227g 4-dimethylaminopyridine (DMAP) into a 100ml single-necked flask, add 30ml of dichloromethane to dissolve, put the single-necked flask into 37 °C in a water bath, stirring evenly. Weigh 0.1917g of 1-(3-dimethylaminopropyl)-3-ethyl...
Embodiment 2
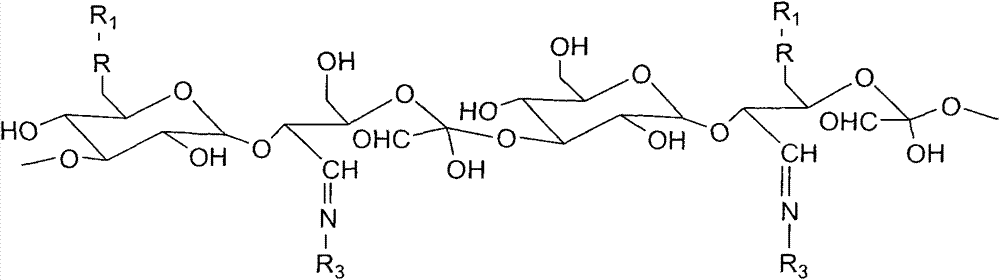
[0034] (4) Synthesis of oxidized dextran
[0035] Dissolve 1g of dextran (Mn 40000) in 30mL of water, add 0.956g of sodium periodate (dissolved in 20mL of water), and react in the dark for 24 hours at room temperature, then add 0.411g of glycerol, continue to stir for 15min, and mix the reaction The solution was transferred to a dialysis bag and dialyzed in deionized water for 24 h, with the water changed every four hours. The dialyzed sample was freeze-dried to obtain the product as a white powder. By changing the molar ratio of dextran and sodium periodate, oxidized dextran with different aldehyde content can be prepared.
[0036] (5) Synthesis of oxidized dextran-RGD
[0037] Add 0.0382g RGD, 0.5g oxidized dextran (oxidation degree 0.5) and 0.1227g 4-dimethylaminopyridine (DMAP) into a 100ml single-necked flask, add 30ml of dichloromethane to dissolve, put the single-necked flask into 60 °C in a water bath, stirring evenly. Weigh 0.1917g of 1-(3-dimethylaminopropyl)-3-e...
Embodiment 3
[0041] (7) Synthesis of oxidized dextran
[0042] Dissolve 1g of dextran (Mn 40000) in 30mL of water, add 0.956g of sodium periodate (dissolved in 20mL of water), and react in the dark for 48 hours at room temperature, add 0.411g of glycerin, continue to stir for 15min, and mix the reaction The solution was transferred to a dialysis bag and dialyzed in deionized water for 48 h, changing the water every four hours. The dialyzed sample was freeze-dried to obtain the product as a white powder. By changing the molar ratio of dextran and sodium periodate, oxidized dextran with different aldehyde content can be prepared.
[0043] (8) Synthesis of oxidized dextran-RGD
[0044] Add 0.0382g RGD, 0.5g oxidized dextran (oxidation degree 0.5) and 0.1227g 4-dimethylaminopyridine (DMAP) into a 100ml single-necked flask, add 30ml of dichloromethane to dissolve, put the single-necked flask into 4 °C in a water bath, stirring evenly. Weigh 0.1917g of 1-(3-dimethylaminopropyl)-3-ethylcarbod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com