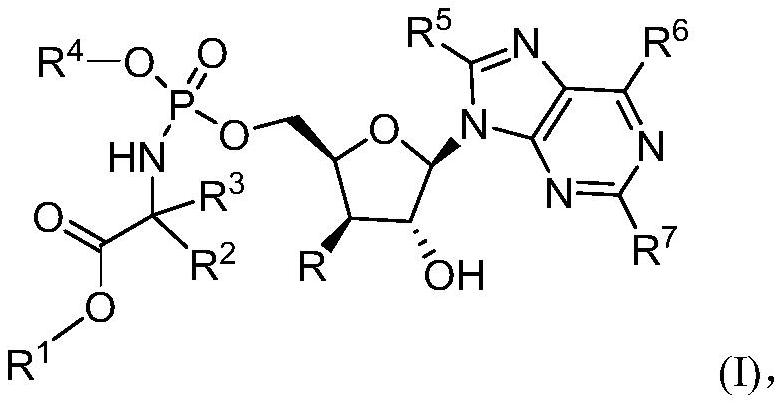
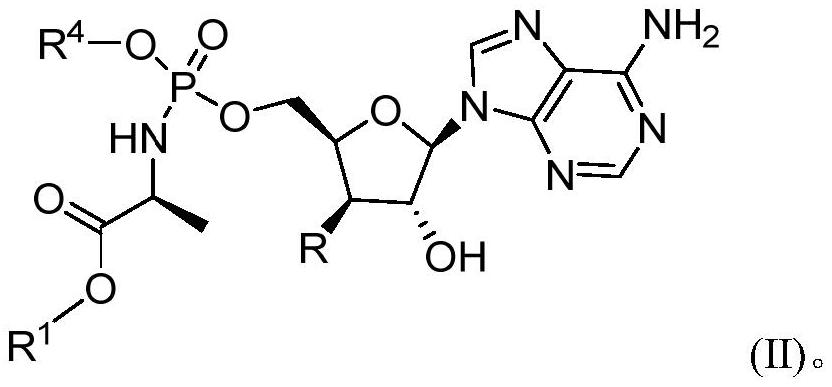
Phosphoramidate derivatives of nucleoside compounds and uses of derivatives
A compound and alkyl technology, applied in the field of compounds and pharmaceutical compositions for the prevention or treatment of viral infections, enteroviridae viral infections, to achieve good metabolic stability, less toxic and side effects, and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
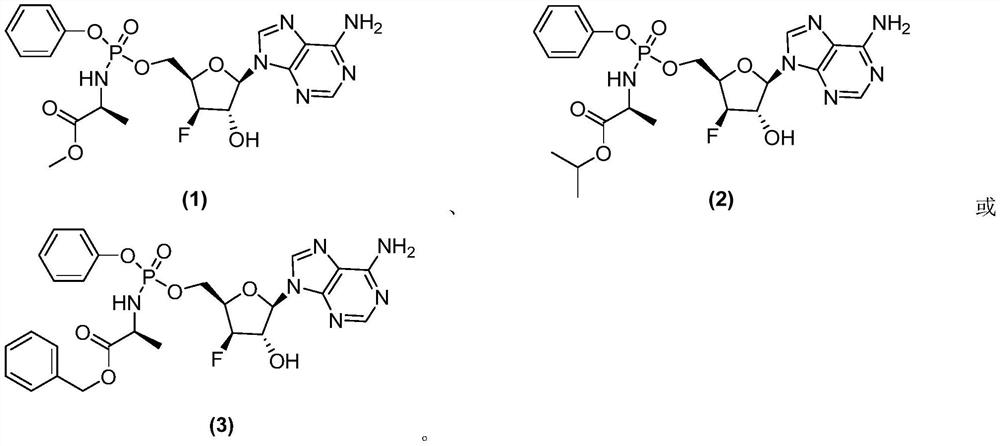
[0167] Example 1 Methyl (((((2R,3R,4S,5R)-5-(6-amino-9H-purin-9-yl)-3-fluoro-4-hydroxytetrahydrofuran-2-yl)methoxy Synthesis of (phenoxy)phosphoryl)-L-alanine ester
[0168]
[0169] Step 1) Synthesis of (chloro(phenoxy)phosphoryl)-L-alanine methyl ester
[0170]
[0171] Weigh (2S)-2-alanine methyl ester hydrochloride (400mg, 2.87mmol) in a 50mL two-necked bottle, add dichloromethane (10 mL) under nitrogen protection and stir at -78°C for 2 minutes, drop Add triethylamine (0.87mL, 6.30mmol), and stir for another two minutes, then dissolve dichlorophosphoryloxybenzene (0.428mL, 2.86mmol) in dichloromethane (10mL), and slowly add it dropwise to the reaction flask, Then keep stirring at -78°C for 30 minutes, warm up to ice bath temperature, and directly use in the next reaction.
[0172] Step 2) Synthesis of ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alanine methyl ester
[0173]
[0174] Under ice bath, dissolve 2,3,4,5,6-pentafluorophenol (527mg, 2.86mmol) and t...
Embodiment 2
[0216] Example 2 Isopropyl (((((2R,3R,4S,5R)-5-(6-amino-9H-purin-9-yl)-3-fluoro-4-hydroxytetrahydrofuran-2-yl)methyl Synthesis of oxy)(phenoxy)phosphoryl)-L-alanine ester
[0217]
[0218]Weigh (2R,3S,4R,5R)-2-(6-amino-9H-purin-9-yl)-4-fluoro-5-(hydroxymethyl)tetrahydrofuran-3-ol (55mg, 0.20mmol) In a 50mL two-necked flask, add N,N-dimethylformamide (3mL) under nitrogen protection, cool to ice bath temperature, add dropwise a tetrahydrofuran solution of tert-butylmagnesium chloride (0.22mL, 0.22mmol, 1.0mol / L) , stirred at room temperature for 10 minutes after dropping, and added ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alanine isopropyl ester (110mg, 0.24mmol) into the reaction flask at ice bath temperature , then returned to room temperature and stirred for 2 hours to stop the reaction, added saturated ammonium chloride aqueous solution (3mL) to quench the reaction, then added ethyl acetate (30mL), washed with water (10mL×3), dried over anhydrous sodium sulfate and conc...
Embodiment 3
[0221] Example 3 Benzyl (((((2R,3R,4S,5R)-5-(6-amino-9H-purin-9-yl)-3-fluoro-4-hydroxytetrahydrofuran-2-yl)methoxy Synthesis of (phenoxy)phosphoryl)-L-alanine ester
[0222]
[0223] Step 1) Synthesis of (chloro(phenoxy)phosphoryl)-L-alanine benzyl ester
[0224]
[0225] Weigh (2S)-2-alanine benzyl ester hydrochloride (800mg, 3.71mmol) in a 50mL two-necked bottle, add dichloromethane (20 mL) under nitrogen protection and stir at -78°C for 2 minutes, drop Add triethylamine (1.10mL, 7.90mmol), and stir for another two minutes, then dissolve dichlorophosphoryloxybenzene (0.55mL, 3.71mmol) in dichloromethane (10mL), and slowly add it dropwise to the reaction flask, Then keep stirring at -78°C for 30 minutes, warm up to ice bath temperature, and directly use in the next reaction.
[0226] Step 2) Synthesis of ((perfluorophenoxy)(phenoxy)phosphoryl)-L-alanine benzyl ester
[0227]
[0228] Under ice bath, dissolve 2,3,4,5,6-pentafluorophenol (682mg, 3.71mmol), triet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com