Preparation method of 3-methyl-2-cyclopentene-1-one
A technology of cyclopentene and methyl, which is applied in the field of preparation of 3-methyl-2-cyclopenten-1-one, can solve the problems of high energy consumption and safety risks, harsh conditions, etc., to reduce energy consumption, The effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
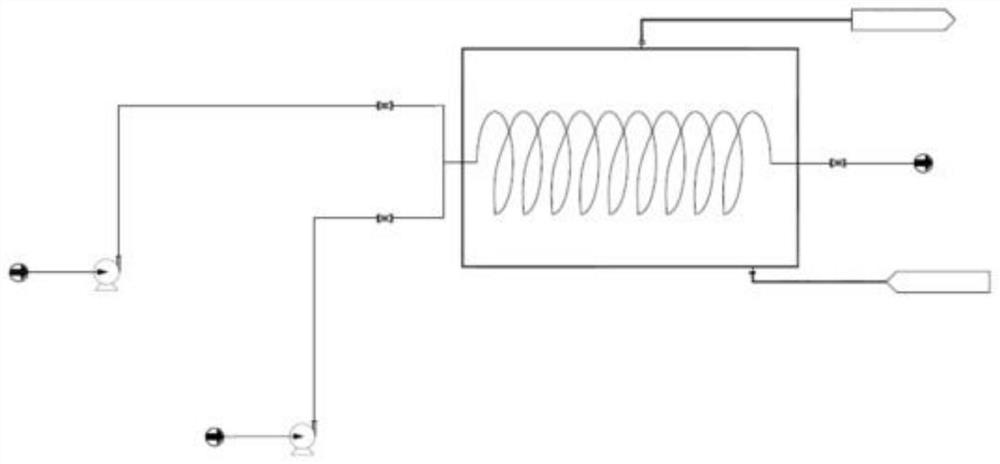
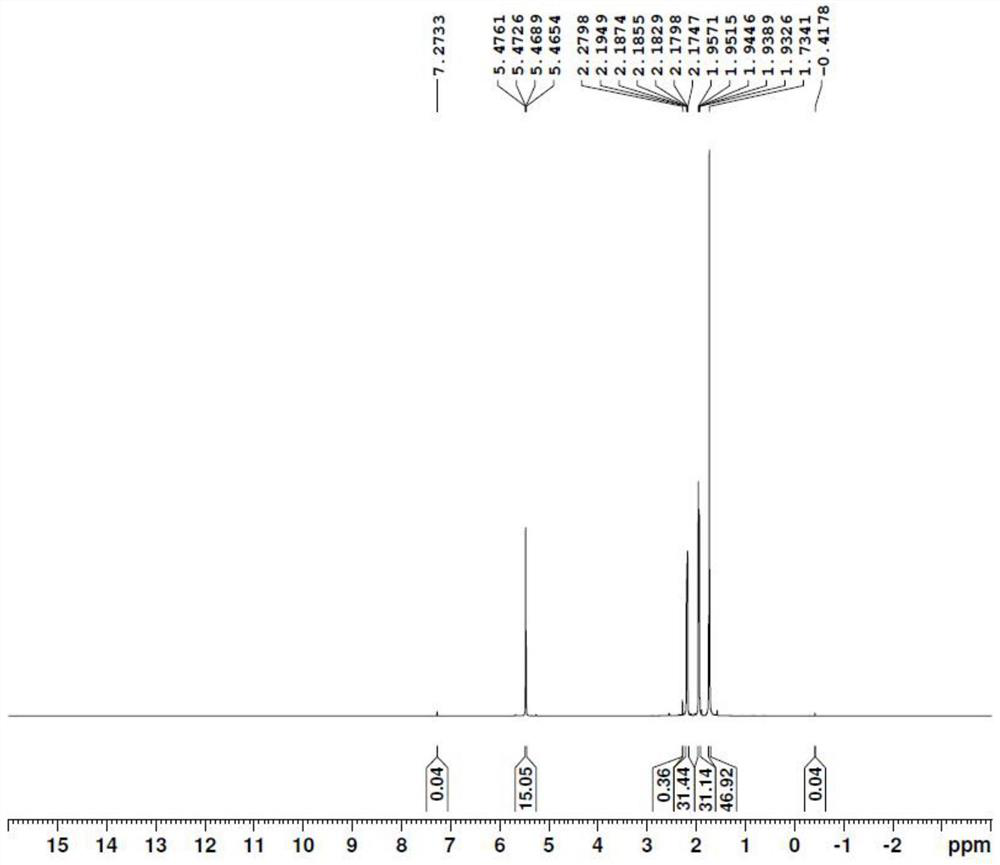
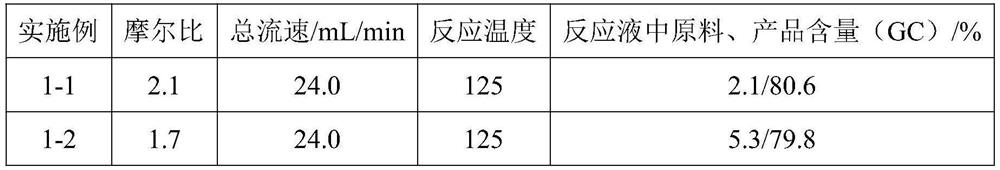
[0029] Add deionized water (300 mL, 2.5 vol) to 2,5-hexanedione (120.0 g), stir well, and record it as solution A. Add deionized water (400 mL) to sodium hydroxide (100.0 g), stir well, and record it as solution B (ie 20% aqueous sodium hydroxide solution). Install the coil reactor, place it in an oil bath, and raise the external temperature to 125°C. Immerse the suction nozzles of plunger pump 1 (hereinafter referred to as pump 1) and plunger pump 2 (hereinafter referred to as pump 2) under the liquid surface of solution A and solution B respectively. Set the flow rate of pump 1 and pump 2 to 12.0mL / min, turn on the two pumps at the same time, and inject solution A and solution B into the reactor from the two feed ports of the reactor for reaction. The effluent from the outlet of the reactor was collected into a 250mL four-necked bottle, and cooled in an external bath at 0-5°C. After receiving the reaction solution for 5 minutes, the reaction was stopped and a sample was ta...
Embodiment 1-2
[0031] Add deionized water (222 mL, 1.85 vol) to 2,5-hexanedione (120.0 g, 1.0 eq), stir well, and record it as solution A. Other conditions are the same as in Example 1-1. After receiving the reaction solution for 5 minutes, the reaction was stopped and a sample was taken, and extracted with ethyl acetate for GC analysis. The molar ratio of sodium hydroxide to 2,5-hexanedione is 1.7:1.
Embodiment 1-3
[0033] Add deionized water (138 mL, 1.15 vol) to 2,5-hexanedione (120.0 g, 1.0 eq), stir well, and record it as solution A. Other conditions are the same as in Example 1-1. After receiving the reaction solution for 5 minutes, the reaction was stopped and a sample was taken, and extracted with ethyl acetate for GC analysis. The molar ratio of sodium hydroxide to 2,5-hexanedione is 1.3:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com