A kind of spinosad tablet and its preparation method and application
A spinosad tablet, spinosad technology, applied in the directions of botanical equipment and methods, application, pesticides, etc., can solve the problems of insect control, inapplicability, difficult mosquito and long-term control, etc., to reduce the environment. The effect of reducing the cost of prevention and control, and improving the efficiency of prevention and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The present embodiment provides a spinosad tablet, which consists of two layers of an immediate-release layer and a sustained-release layer. The total content of the spinosad in the immediate-release layer and the sustained-release layer is 7.5%, and the tablet weight is 1.33g. The immediate release layer is 0.2g, and the sustained release layer is 1.13g. The formulations of the immediate-release layer and the sustained-release layer per 100 parts are as follows:
[0048] Immediate release layer (100 parts): 5 parts of spinosad, 10 parts of PEG, 7 parts of sodium bicarbonate, 7 parts of citric acid, 1 part of sodium polyphosphate, 1 part of lignosulfonate, 0.8 parts of polyvinylpyrrolidone, Carboxymethyl starch sodium 2 parts, lactose added to 100 parts;
[0049] Sustained release layer (100 parts): spinosad 8 parts, calcium sulfate 24 parts, PEG 7 parts, naphthalene sulfonate 1 part, lignosulfonate 1 part, polyvinylpyrrolidone 0.8 part, white carbon black 2 parts , s...
Embodiment 2
[0053] The present embodiment provides a spinosad tablet, which is composed of two layers of an immediate-release layer and a sustained-release layer, the total content of the spinosad in the immediate-release layer and the sustained-release layer is 6.25%, and the tablet weight is 1.33g. The immediate release layer is 0.2g, and the sustained release layer is 1.13g. The formulations of the immediate-release layer and the sustained-release layer per 100 parts are as follows:
[0054] Immediate release layer (100 parts): 2 parts of spinosad, 10 parts of PEG, 7 parts of sodium bicarbonate, 7 parts of citric acid, 1 part of sodium polyphosphate, 1 part of lignosulfonate, 0.7 parts of polyvinylpyrrolidone, Carboxymethyl starch sodium 2 parts, lactose added to 100 parts;
[0055] Sustained release layer (100 parts): spinosad 7 parts, calcium sulfate 14 parts, PEG 7 parts, naphthalene sulfonate 1 part, lignosulfonate 1 part, polyvinylpyrrolidone 0.8 part, white carbon black 2 parts ...
experiment example 1
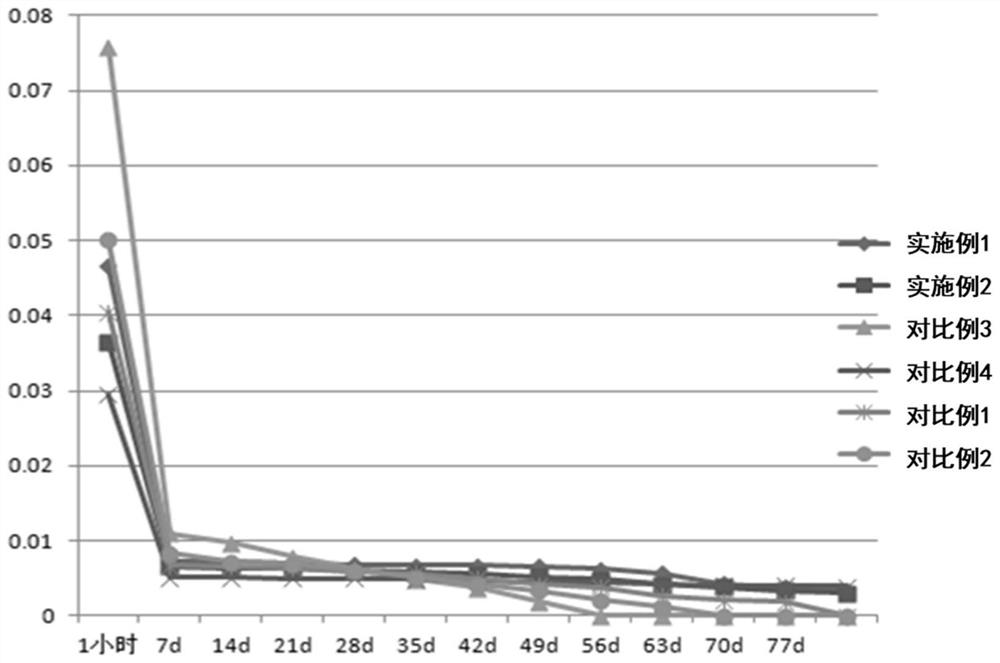
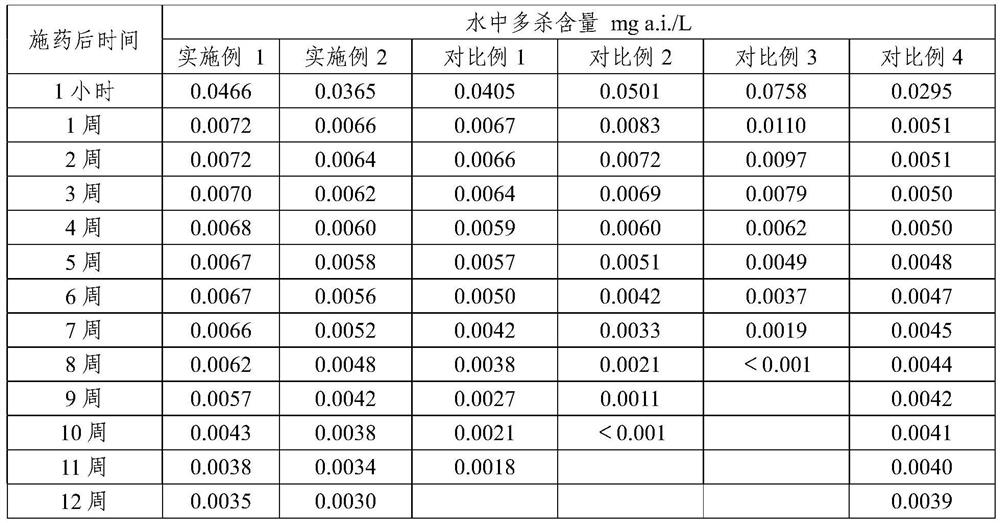
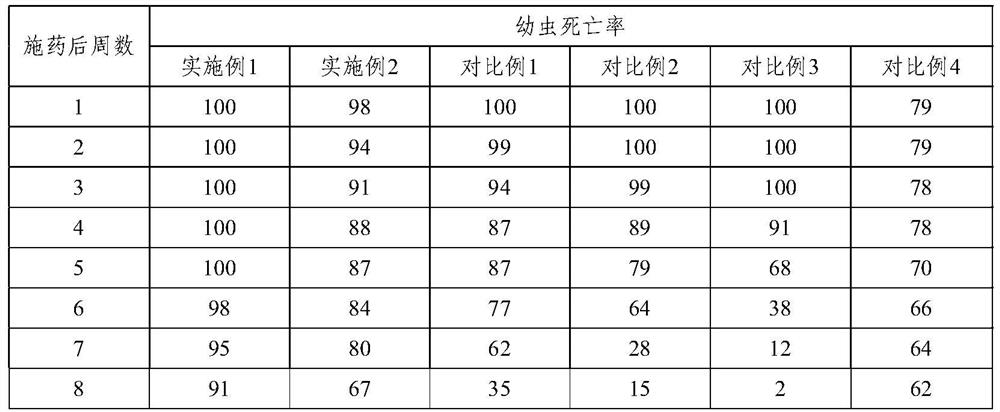
[0068] The release analysis of experimental example 1 spinosad tablet
[0069] The spinosad tablets of Examples 1~2 and Comparative Examples 1~4 are placed in water, utilize HPLC to detect the content of spinosad at different times, analyze the release rate of spinosad tablets over time, the result As shown in Table 1 and figure 1 shown.
[0070] The release rate of table 1 spinosyn tablet
[0071]
[0072] The results show that, adopting the slow-release carriers of Comparative Examples 1 and 2, the slow-release effect of spinosad tablets is significantly reduced, wherein, only calcium sulfate is used as the slow-release carrier, the initial spinosad releases faster, and the spinosad The release of spinosad into the water is premature, and the content in the water is very low in the later stage, and the insecticidal effect is greatly reduced; the release rate of calcium sulfate combined with stearic acid is slow, and spinosad is partially decomposed in water, and the ins...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com