2, 5-bis (2, 6-difluorobenzylidene)-cyclopentanone and preparation method and application thereof
A technology of difluorobenzylidene and cyclopentanone, which is applied in the direction of condensation preparation of carbonyl compounds, digestive system, endocrine system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides the preparation method of the 2,5-bis(2,6-difluorobenzylidene)-cyclopentanone, see Figure 6 , including the following steps:
[0034] Mix cyclopentanone with the structure shown in formula II and 2,6-difluorobenzaldehyde with the structure shown in formula III in a solvent, use sodium methoxide as a catalyst, and generate aldehydes and ketones at 20-30°C Condensation reaction, the reaction lasts for 12 to 16 hours, and the reactant is separated and purified to obtain a yellow powder product;
[0035]
[0036] In the present invention, the molar mass ratio of cyclopentanone, 2,6-difluorobenzaldehyde and sodium methoxide is preferably 1:18-22:24-28, more preferably 1:20:26. The solvent is preferably ethanol or methanol.
[0037] In the present invention, the separation and purification method of the reactant is preferably to filter the obtained reactant in ice water, collect the retentate, wash with water, and evaporate to dryness under reduce...
Embodiment 1
[0044] Dissolve 2.08 mmol of cyclopentanone with the structure of formula II in 100 mL of absolute ethanol, stir at room temperature for 5 minutes, then add 42.2 mmol of 2,6-difluorobenzaldehyde with the structure of formula III, and continue stirring for 10 minutes, the solution remains unchanged. Continue to slowly drop 15 mL of sodium methoxide (NaOMe) solution (containing 54.8 mmol of sodium methoxide) into the reaction solution, stir at 25° C. for 14 h, pour the mixture into ice water (150 mL) and filter. After filtration, it was washed twice with water (20 mL) and evaporated to dryness under reduced pressure. followed by CH 2 Cl 2 or CH 3 Wash twice with OH (20 mL), and dry in vacuo at 30°C to obtain a yellow powder product, which is purified by silica gel column chromatography to obtain a compound of formula I with a purity greater than 79.2%;
[0045]
[0046] The compound of formula I is subjected to structural characterization, and the results are as follows: ...
Embodiment 2
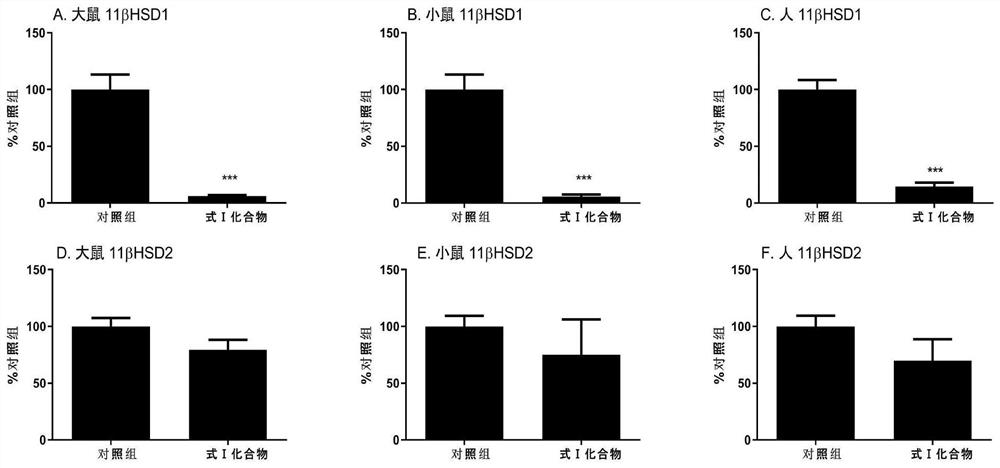
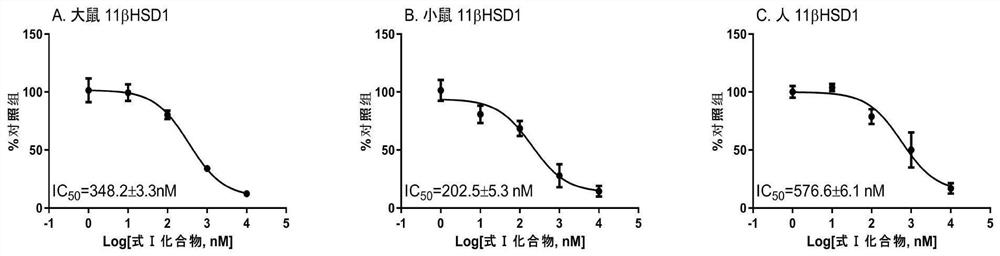
[0048] Compounds with Formula I Selectively Inhibit 11β-Hydroxysteroid Dehydrogenase 1 Activity Experiment
[0049] Human kidney and liver microsomes were purchased from a genetic testing company, and rat and mouse kidney and liver microsomes were directly prepared and extracted (Guo, J., Yuan, X., Qiu, L., Zhu, W., Wang, C., Hu, G., Chu, Y., Ye, L., Xu, Y., and Ge, R.S., 2012. Inhibition of human and rat 11beta-hydroxysteroid dehydrogenases activities by bisphenol A. Toxicol Lett 215, 126-130).
[0050] Treat the liver and kidney microsomes (10 μg) of rats, mice and people with the formula I structural compound prepared in 100 μM embodiment 1 for 30 minutes, then use radioimmunoassay technique (Ge, R.S., Dong, Q., Niu, E .-M.,Sottas,C.M.,Hardy,D.O.,Catterall,J.F.,Latif,S.A.,Morris,D.J.,and Hardy,M.P.,2005.11b-Hydroxysteroid dehydrogenase 2 in rat Leydig cells:its role in bluntingglucocorticoid action at physiological le substrate. Endocrinology 146, 2657-2664) was used to de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com