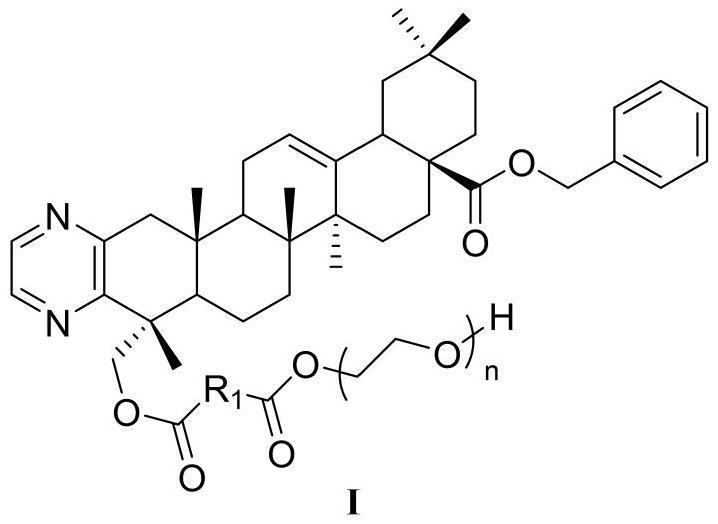
Application of hederagenin polyethylene glycol derivative
A technology of helexin and polyethylene glycol is applied in the directions of medical preparations, drug combinations, steroids, etc. containing active ingredients, which can solve the problems of low solubility, improve water solubility, and reverse the resistance of excellent tumor resistance. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0042] Example 14-(23-oxyl olean-12-en-28-acid benzyl[3,2-b]pyrazine)-4-oxo-butyric acid triethylene glycol ester (H6 -D-PEG 200 ) synthesis and characterization
[0043] The compound helexin (472.0mg, 1.0mmol) was dissolved in N,N-dimethylformamide (15.0mL), potassium carbonate (300.0mg, 2.1mmol), benzyl bromide (0.2mL, 1.3mmol) were added , Stir at 50°C for 6-10h. The reaction solution was diluted with ethyl acetate (25.0 mL), washed three times with water, washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, the solvent was evaporated under reduced pressure, and silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =10:1-5:1), a white solid (470.0 mg, 83.0%) was obtained.
[0044] The above compound (460.0 mg, 0.8 mmol) was dissolved in 20.0 mL of dichloromethane, 4-dimethylaminopyridine (122.0 mg, 1.0 mmol) and tert-butyldimethylsilyl chloride (360.0 mg, 2.4 mmol) were added, Stir at room temperature for 4-8h. Dichloromethane was distilled off,...
Embodiment 21
[0050] Example 21'-(23-Oloxyolean-12-ene-28-acid benzyl[3,2-b]pyrazine)-1'-propionyl-3,3'-dithiopropane Acetate triethylene glycol ester (H6-S-PEG 150 ) synthesis and characterization
[0051] H6 (100.0 mg, 0.2 mmol) was dissolved in anhydrous dichloromethane (8.0 mL), the catalyst DMAP (102.3 mg, 0.8 mmol), EDCI (160.6 mg, 0.8 mmol) and 3,3'-dithio Dipropionic acid (352.3mg, 1.7mmol) was refluxed at 40°C for 7h. After the reaction, dichloromethane was added for dilution, and the organic layer was washed once with 5% HCl solution, twice with deionized water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a white solid H6- S (121.9 mg, 92.2%).
[0052] Dissolve triethylene glycol (131.7 μL, 1.1 mmol) in anhydrous dichloromethane (8.0 mL), add H6-S (88.0 mg, 0.1 mmol), catalyst DMAP (129.4 mg, 1.1 mmol), EDCI (128.3mg, 0.7mmol), stirred at room temperature for about 10h. After the reaction finished, the solve...
Embodiment 31
[0053] Example 31'-(23-Oxylean-12-en-28-acid benzyl[3,2-b]pyrazine)-1'-acetyl-2,2'-oxoacetic acid poly Ethylene glycol (600) ester (H6-E-PEG 600 ) synthesis and characterization
[0054] Dissolve H6 (70.0mg, 0.1mmol) in anhydrous dichloromethane (8.0mL), add catalyst DMAP (71.6mg, 0.6mmol) and diglycolic anhydride (68.0mg, 0.6mmol), react at room temperature for 0.5h . After the reaction, dichloromethane was added for dilution, and the organic layer was washed once with 10% HCl solution, twice with deionized water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain white solid H6- E (78.0 mg, 93.3%).
[0055] Dissolve polyethylene glycol 600 (543.4 μL, 1.2 mmol) in anhydrous dichloromethane (8.0 mL), add H6-E (82.0 mg, 0.1 mmol), catalyst DMAP (133.5 mg, 1.1 mmol), EDCI ( 132.3mg, 0.7mmol), stirred at room temperature for about 10h. After the reaction finished, the solvent was removed by distillation under redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com