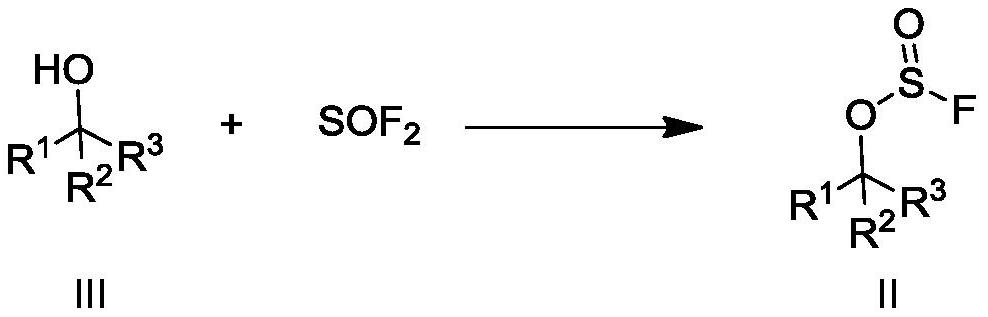
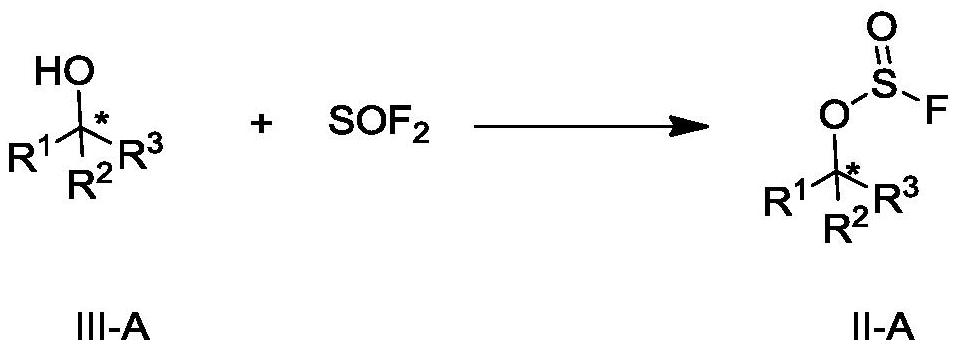
Preparation method of fluoride and intermediate thereof
A compound, thionyl fluoride technology, applied in the field of preparation of fluoride and its intermediates, can solve the problems of strong corrosion, cumbersome operation, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] [chemical formula 3]
[0125]
[0126] Select optically active [chemical formula 3] α-hydroxy carboxylate methyl ester as substrate, in the reactor, charge the [chemical formula 3] α-hydroxy carboxylate of 10.6g (100mmol, 1.00eq, photochemical purity more than 98.0%ee) Acetate methyl ester, and 11.1g (110mmol, 1.1eq) of triethylamine, after adding 100ml of solvent DMF (N,N-dimethylamide) and stirring at -15°C, then slowly bubbling 9.89g of (115mmol, 1.15eq) of SOF 2 Gas (thionyl fluoride gas), the internal temperature will increase with the amount of gas blown in. After 1h, intermediate II-1 is obtained. After testing, 19 FNMR (376MHz, reference substance is trifluorotoluene) δ62.11, 60.32 (two fluorine peaks).
[0127]
[0128] The mixed solution was heated to 100° C., and the reaction was completed after 2 hours, and the reaction conversion rate reached more than 95%. Then, the reaction-terminated liquid was directly distilled under reduced pressure, and the ...
Embodiment 2
[0145] Select optically active α-hydroxycarboxylate methyl ester as substrate, and charge [chemical formula 3] α-hydroxycarboxylate methyl ester and 5.55g (50mmol, 0.5eq) of triethylamine, then add 100ml of solvent DMF (N,N-dimethylamide) and stir evenly at -15°C, then slowly bubble 9.89g (115mmol, 1.15eq ) of SOF 2 Gas (thionyl fluoride gas), the internal temperature will increase with the amount of gas blown in. After 1 hour, the mixed solution is heated to 100 ° C. After 2 hours of reaction, the reaction conversion rate reaches more than 95%.
[0146] Then, the reaction-terminated liquid was directly distilled under reduced pressure, and the temperature of the distilled oil bath was 80° C., thereby obtaining 6.3 g of liquid (R)-methyl 2-fluoropropionate fraction product of [Chemical Formula 4]. The recovery rate was 60%. The chemical purity of the distillate was 1 The photochemical purity was above 95% as calculated by H NMR.
[0147] (R)-Methyl 2-fluoropropionate 19 F...
Embodiment 3
[0149]Select optically active [chemical formula 3] α-hydroxy carboxylate methyl ester as substrate, in the reactor, charge the [chemical formula 3] α-hydroxy carboxylate of 10.6g (100mmol, 1.00eq, photochemical purity more than 98.0%ee) Acetate methyl ester and 11.1g (110mmol, 1.1eq) of triethylamine, after adding 100ml of solvent DMF (N,N-dimethylamide) and stirring at -15°C, then slowly bubbling 9.89g ( 115mmol, 1.15eq) of SOF 2 Gas (thionyl fluoride gas), the internal temperature will increase with the amount of gas blown in. After 1 hour, the mixed solution is heated to 100 ° C. After 2 hours of reaction, the reaction conversion rate reaches more than 95%.
[0150] Then, the reaction-terminated liquid was directly distilled under reduced pressure, and the temperature of the distilled oil bath was 80° C., thereby obtaining 7.3 g of liquid (R)-methyl 2-fluoropropionate fraction product of [Chemical Formula 4]. The recovery rate was 65%. The chemical purity of the distillat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com