Method for catalyzing ring-opening polymerization of cyclic ester by zinc catalyst
A technology of ring-opening polymerization and zinc catalyst, which is applied in the field of polymer synthetic materials, and achieves the effect of wide application prospects, different structures and properties, and good monomer universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
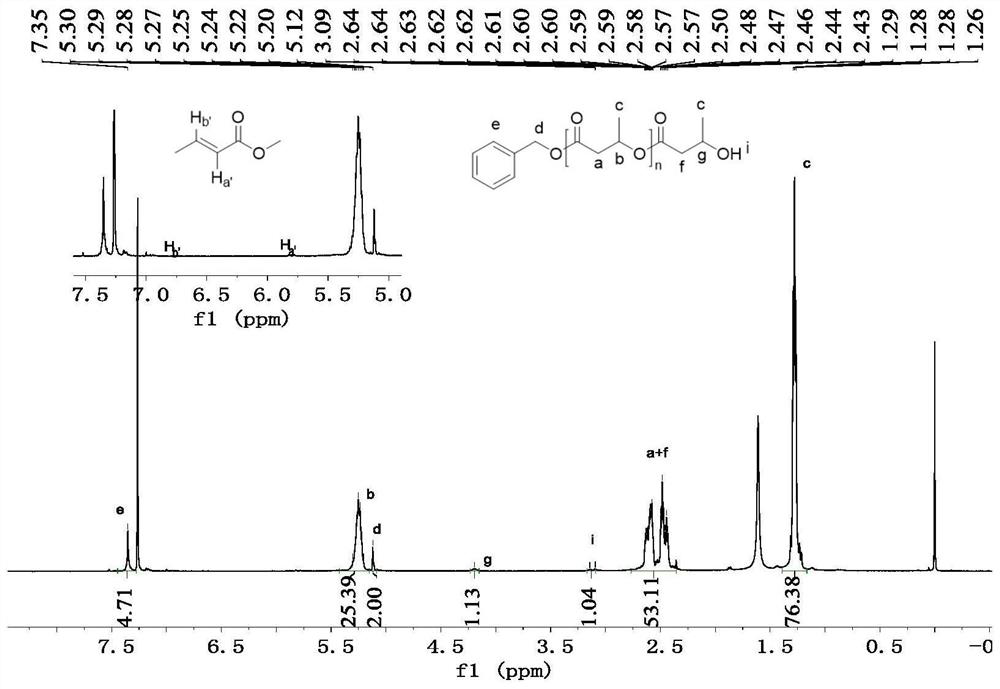
[0029] Example 1: Catalytic ring-opening polymerization of racemic lactide under different addition amounts of alcohol initiators.
[0030] 1. Take a 5mL Schlenk bottle, roast it and replace the argon, then add 3.86mg (10μmol, 1eq.) of Zn[N(SiMe 3 ) 2 ] 2 The catalyst was dissolved in 1 mL of solvent, and then 1 mol / L toluene solution of different equivalents of benzyl alcohol was added, and stirred for five minutes. Then 144 mg (1 mmol, 100 eq.) of racemic lactide monomer was added, and the reaction was stirred at room temperature.
[0031] 2. After the reaction finishes, benzoic acid quenches the reaction. The conversion rate of the reaction was determined by NMR. The solvent was removed in vacuo, the polymer was isolated by washing with ice methanol, and dried in vacuo to constant weight. The obtained polymer measures the molecular weight (Mn) and the molecular weight distribution (PDI) of the polymer by gel permeation chromatography (GPC), and calculates the isotactic...
Embodiment 2
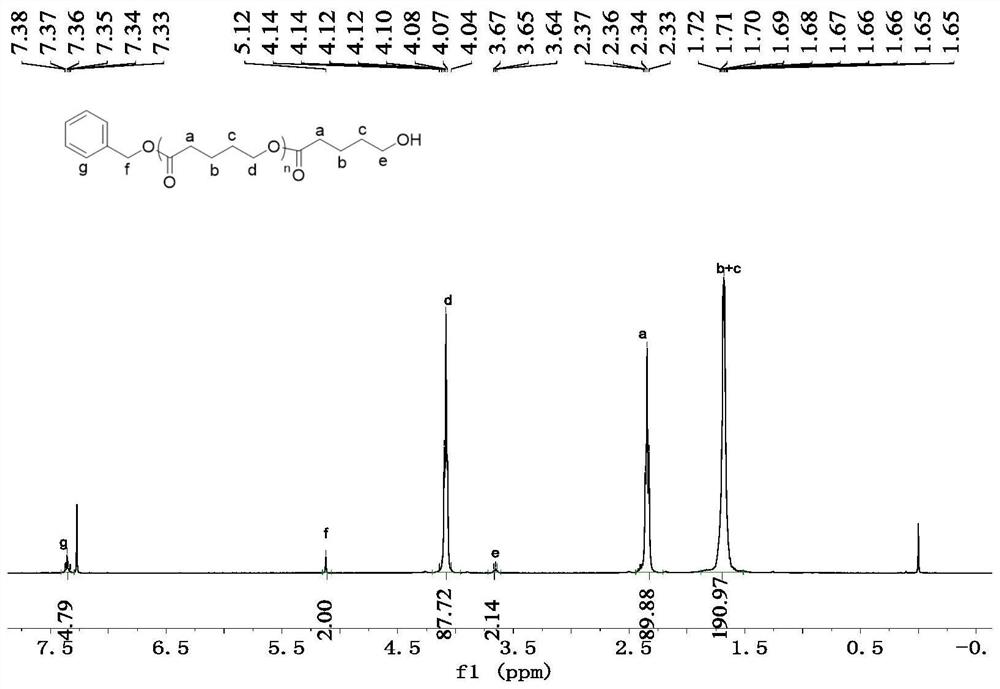
[0035] Example 2: Ethyl lactate initiated ring-opening polymerization of racemic lactide.
[0036] 1. Take a 5mL Schlenk bottle, roast it and replace the argon, then add 3.86mg (10μmol, 1eq.) of Zn[N(SiMe 3 ) 2 ] 2 The catalyst was dissolved in 1 mL of toluene solvent, and then 40 μL of 1 mol / L toluene solution of ethyl lactate was added, and stirred for five minutes. Then 144 mg (1 mmol, 100 eq.) of racemic lactide monomer was added, and the reaction was stirred at room temperature.
[0037] 2. After reacting for 8 minutes, benzoic acid quenched the reaction. The conversion rate of the reaction determined by NMR was 97%. The solvent was removed in vacuo, the polymer was isolated by washing with ice methanol, and dried in vacuo to constant weight. The molecular weight (Mn) of the obtained polymer measured by gel permeation chromatography (GPC) is 9400 g / mol and the molecular weight distribution (PDI) is 1.26, and the isotacticity calculated by homonuclear decoupling NMR s...
Embodiment 3
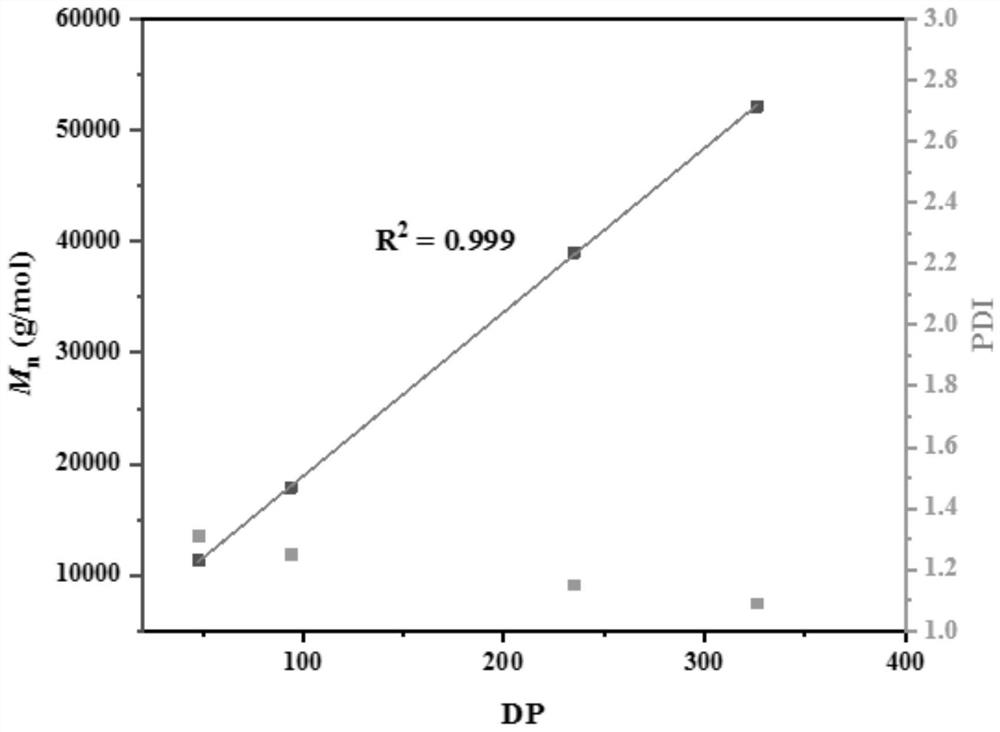
[0038] Example 3: Catalytic ring-opening polymerization of racemic lactide under different monomer equivalents.
[0039] 1. Take a 10mL Schlenk bottle, pump and bake it and replace the argon, then add 3.86mg (10μmol, 1eq.) of Zn[N(SiMe3 ) 2 ] 2 The catalyst was dissolved in 1 mL of dichloromethane solvent, then 20 μL of benzyl alcohol in 1 mol / L toluene solution was added, and stirred for five minutes. Then add racemic lactide monomers with different equivalent ratios, control the monomer concentration to 1 mol / L, add a solvent to dissolve the monomers, and stir at room temperature for reaction.
[0040] 2. After the reaction finishes, benzoic acid quenches the reaction. The conversion rate of the reaction was determined by NMR. The solvent was removed in vacuo, the polymer was isolated by washing with ice methanol, and dried in vacuo to constant weight. The obtained polymer measures the molecular weight (Mn) and the molecular weight distribution (PDI) of the polymer by ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com