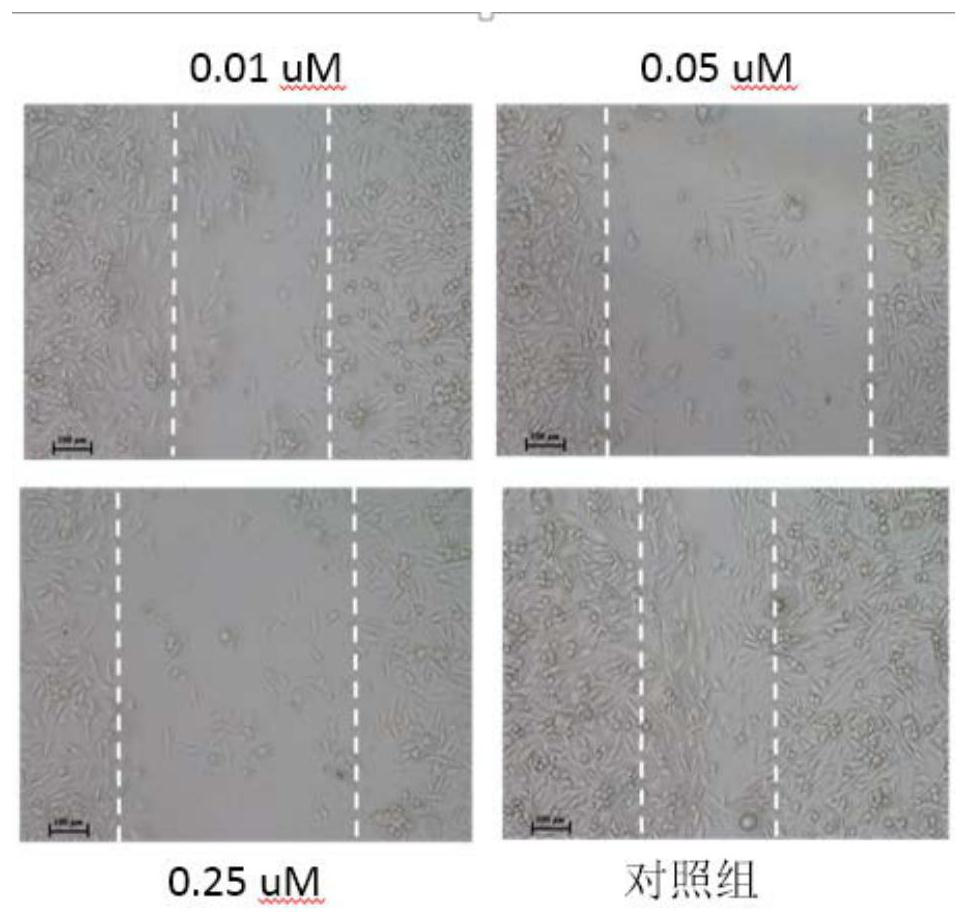
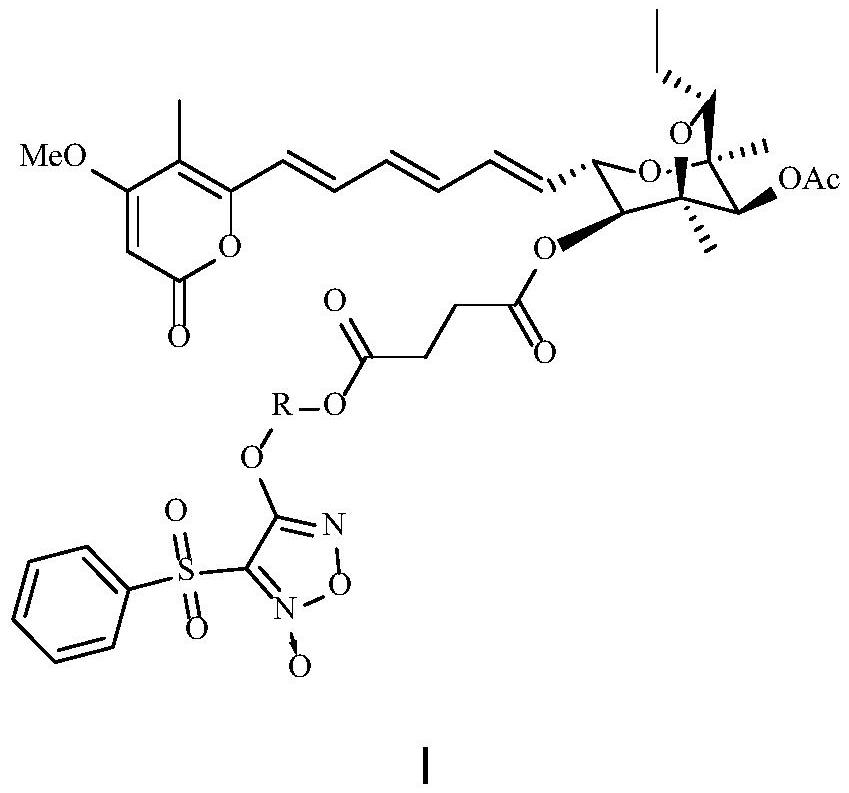
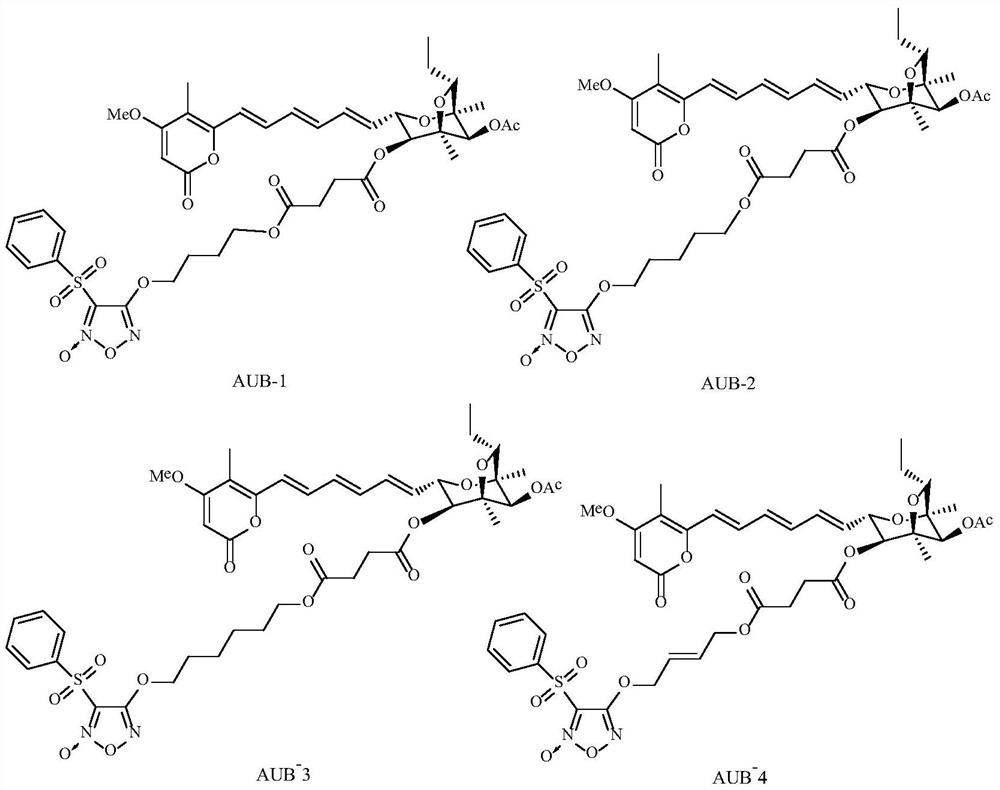
Aurovertin B derivative as well as preparation method and application thereof
A derivative and product technology, applied in the field of medicine, can solve the problems of insufficient activity and specificity of triple-negative breast cancer, and achieve the effects of improving druggability, good application prospects, and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthesis of compound described in formula II
[0046] Weigh 4.75g (0.028mol) of phenylthioacetic acid into a round bottom flask, add 30ml of acetic acid, stir on a stirrer until completely dissolved, slowly add 30% H 2 o 2 7ml (0.069 mol), the ice bath was removed after feeding, and the reaction was stirred at room temperature for 3 hours, and the progress of the reaction was detected by pointing a plate during the process. After the reaction of the raw materials is complete, slowly add 12.5ml (0.300 mol) of fuming nitric acid under the condition of ice bath at 0°C, and reflux at 110°C for 2 hours. Needle-like crystals were precipitated, filtered, and the filtered solid was washed three times with a small amount of water, and vacuum-dried to obtain the target product, a total of 2.29 g. The yield was 44.3% based on the amount of phenylthioacetic acid.
Embodiment 2
[0047] Example 2: Synthesis of aurovertin B derivative AUB-1 (the synthetic route is shown in formula 1)
[0048]
[0049] Weigh the compound described in Formula II (144 mg, 0.39 mmol) into a round bottom flask, add 4 mL of THF, select a moderate stirring magnet, stir on a stirrer to dissolve it, then add 0.4 mL of NaOH aqueous solution (36.8 mg, 0.92 mmol ), and added 1,4-butanediol (0.63mmol), and reacted at room temperature for 3h. During the process, TLC monitored the reaction process. After the raw materials were completely converted, 4mL of distilled water was added to complete the reaction. The reaction solution was extracted three times with 5mL of ethyl acetate. After liquid separation, the organic layer was taken and spin-dried, and separated by silica gel column chromatography (200 mesh-300 mesh), the eluent was petroleum ether: acetone = 3.5:1, and a thin layer of silica gel was used for detection. The eluate with strong ultraviolet absorption spots under the l...
Embodiment 3
[0053] Example 3: Synthesis of aurovertin B derivative AUB-2 (the synthetic route is shown in Formula 1)
[0054]
[0055] Weigh the compound described in Formula II (144 mg, 0.39 mmol) into a round bottom flask, add 4 mL of THF, select a moderate stirring magnet, stir on a stirrer to dissolve it, then add 0.4 mL of NaOH aqueous solution (36.8 mg, 0.92 mmol ), and added 1,5-pentanediol (0.63mmol), and reacted at room temperature for 3h. During the process, TLC monitored the reaction process. After the raw materials were completely converted, 4mL of distilled water was added to complete the reaction. The reaction solution was extracted three times with 5mL of ethyl acetate. After liquid separation, the organic layer was taken and spin-dried, and separated by silica gel column chromatography (200 mesh-300 mesh), the eluent was petroleum ether: acetone = 3.5:1, and a thin layer of silica gel was used for detection. The eluate with strong ultraviolet absorption spots under the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com