Energy-efficient system and method for carbon dioxide conversion
A carbon dioxide and conversion system technology, applied in the direction of carbon monoxide, chemical instruments and methods, and hydrocarbon production from carbon oxides, to achieve the effect of simplifying the reaction system and improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
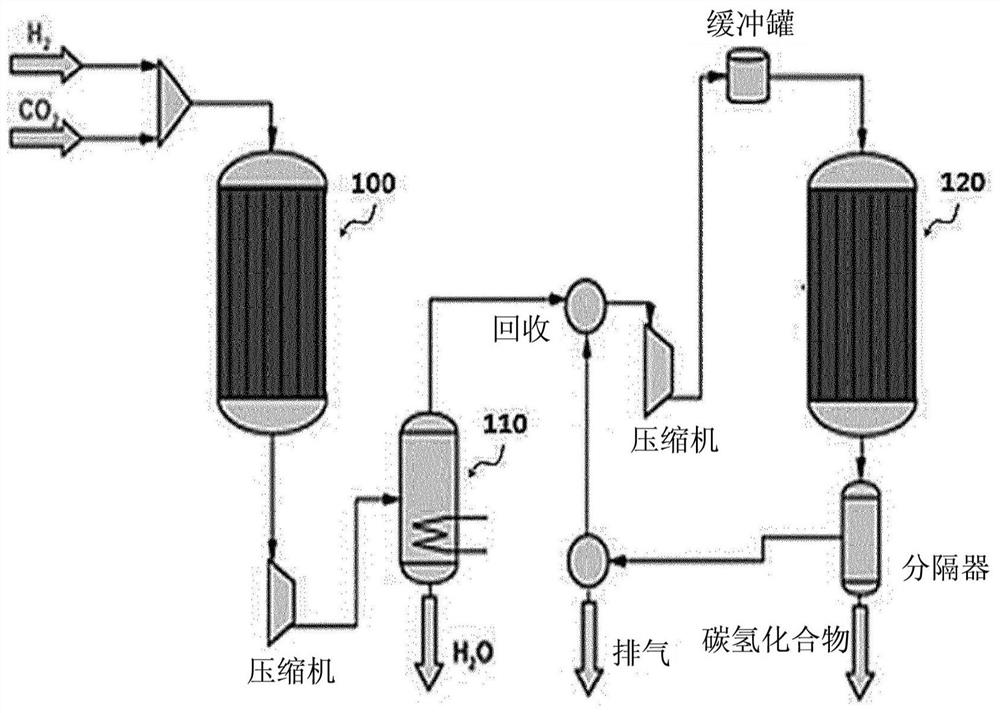
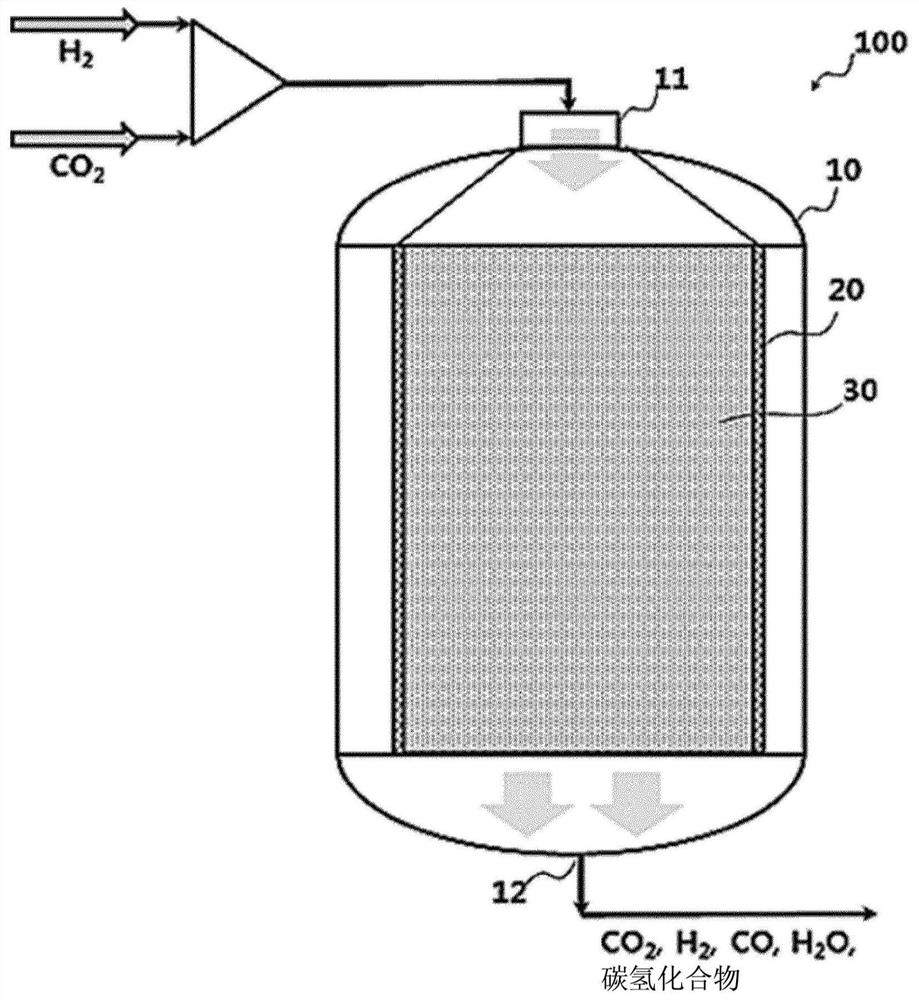
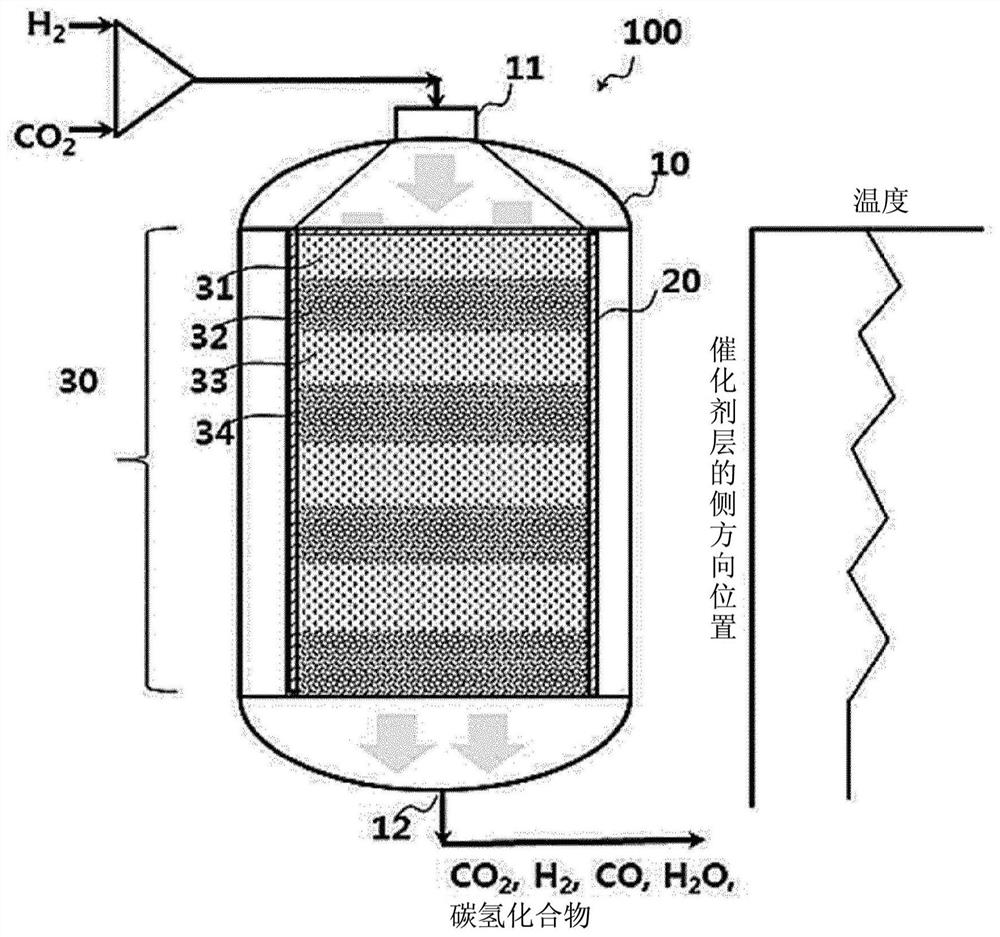
[0090] First, the influence of the mixing ratio of the catalyst for the reverse water gas shift reaction (RWGS) and the catalyst for the Fischer-Tropsch synthesis reaction (FTS) will be observed. In the catalyst layer of 0.4g mass in total, weigh 0.3g as FTS catalyst and contain the mixed catalyst of 100 parts by weight of Fe, 13 parts by weight of Cu, 12 parts by weight of Al, K15 parts by weight (marked as 100Fe-13Cu- 12Al-15K), then weigh out 0.1g in Ce 2 The RWGS catalyst loaded with 1 wt% Pt in the O support (hereinafter denoted as 1Pt-Ce 2 O), and uniformly mix the two catalysts. Mix the above two types of catalysts evenly into 2 g of diluent (α-alumina) and follow as follows figure 2 The manner shown was filled into a 1 / 2 inch stainless steel fixed bed reactor (carbon monoxide generation section). Before starting the reaction, the catalyst filled into the reactor was heated at 400°C with 2000mlh -1 gcat -1 The flow rate of hydrogen was used for 2 hours of reductio...
Embodiment 2
[0092] In a total mass of 0.4g of the catalyst layer, 0.2g of FTS catalyst with a composition ratio of 100Fe-13Cu-12Al-15K and 0.2g of a composition ratio of 1Pt-Ce 2 O's RWGS catalyst was mixed. The carbon dioxide conversion reaction was carried out under the same remaining conditions as in Example 1 above, and the results are shown in Table 1.
Embodiment 3
[0094] 0.1 g of an FTS catalyst having a composition ratio of 100Fe-13Cu-12Al-15K and 0.3 g of a RWGS catalyst having a composition ratio of 1Pt-Ce2O were mixed in a total mass of 0.4 g of the catalyst layer. The carbon dioxide conversion reaction was carried out under the same remaining conditions as in Example 1 above, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com