Metal oxide energy storage material with special microstructure and preparation method
A technology of microstructure and energy storage materials, applied in the field of materials, can solve the problems of limiting the potential of industrial applications, complicated preparation processes, and consumption of lithium sources, so as to improve high-rate working performance, high cycle stability, and reduce grain boundaries The effect of resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
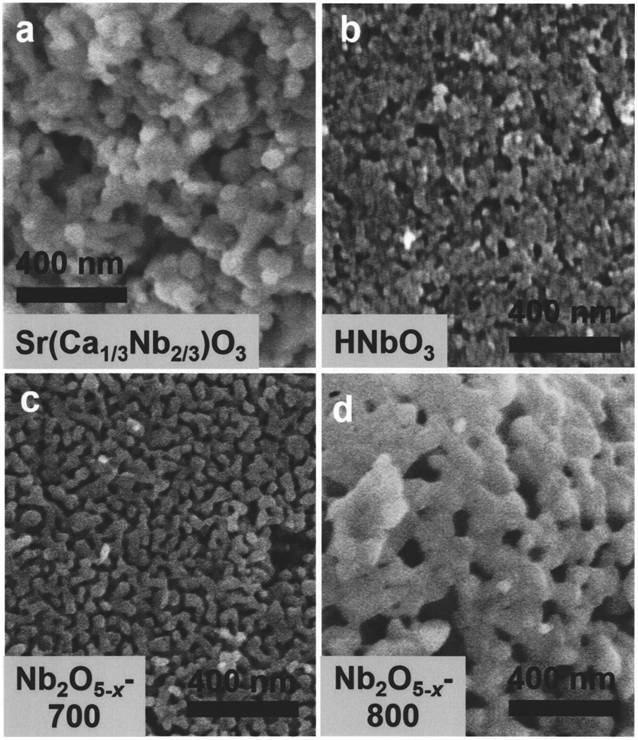
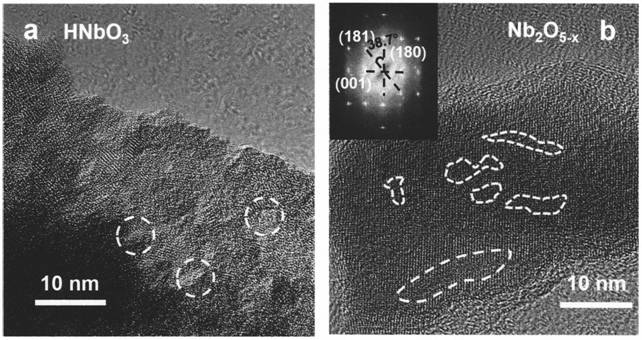
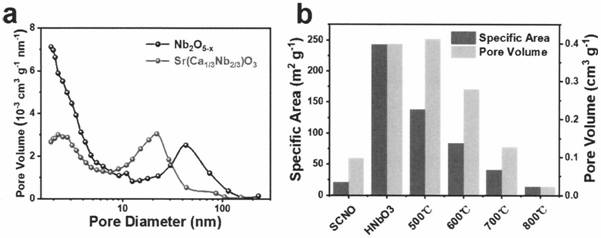
[0024] SrCa synthesized by sol-gel method 1 / 3 Nb 2 / 3 o 3 Add 2 times the molar ratio of hydrochloric acid aqueous solution to the precursor, keep stirring at 80°C for two days, filter the particles, add 2 times the molar ratio of hydrochloric acid aqueous solution, and place the mixed system in a high-temperature and high-pressure reaction vessel, and react at 130°C After three days, filter the particles, and finally add an aqueous hydrochloric acid solution with a molar ratio of 2 times, and place the mixed system in a high-temperature and high-pressure reaction vessel, and react at 160°C for three days to obtain an intermediate product. During the reaction, all the strontium ions, calcium ions and part of the niobium ions in the raw materials were dissolved by the hydrochloric acid solution, and the hydrogen ions in the hydrochloric acid solution were exchanged into the crystal structure. After the reaction was completed, the precipitate was separated by filtration under re...
Embodiment 2
[0026] LiNbO synthesized by sol-gel method 3 Add 3 times the molar ratio of hydrochloric acid aqueous solution, stir at 80°C and keep warm for two days, filter the particles, add 3 times the molar ratio of hydrochloric acid aqueous solution, and place the mixed system in a high-temperature and high-pressure reaction vessel, and react at 110°C for three One day, filter the particles, and finally add a 3-fold molar ratio of hydrochloric acid aqueous solution, and place the mixed system in a high-temperature and high-pressure reaction vessel, and react at 150°C for three days to obtain an intermediate product. During the reaction, all the lithium ions and part of the niobium ions in the raw material are dissolved by the hydrochloric acid solution, and the hydrogen ions in the hydrochloric acid solution are exchanged into the crystal structure. After the reaction is completed, the precipitate is separated by filtration under reduced pressure, and the white intermediate product is n...
Embodiment 3
[0028] SrMoO synthesized by sol-gel method 4 Add 5 times the molar ratio of hydrochloric acid aqueous solution, stir at 80°C and keep warm for two days, filter the particles, add 5 times the molar ratio of hydrochloric acid aqueous solution, and place the mixed system in a high temperature and high pressure reaction vessel, and react at 110°C for three One day, filter the particles, and finally add a 5-fold molar ratio of hydrochloric acid aqueous solution, and place the mixed system in a high-temperature and high-pressure reaction vessel, and react at 200°C for three days to obtain an intermediate product. During the reaction, all the strontium ions and part of the molybdenum ions in the raw materials are dissolved by the hydrochloric acid solution, and after the reaction is completed, the precipitates are separated by filtration under reduced pressure, and the white intermediate product is molybdenum oxide particles with an amorphous structure. The prepared particles were ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com