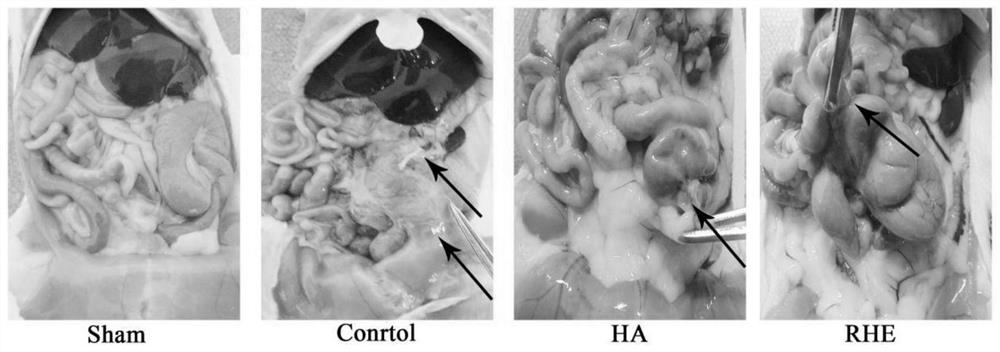
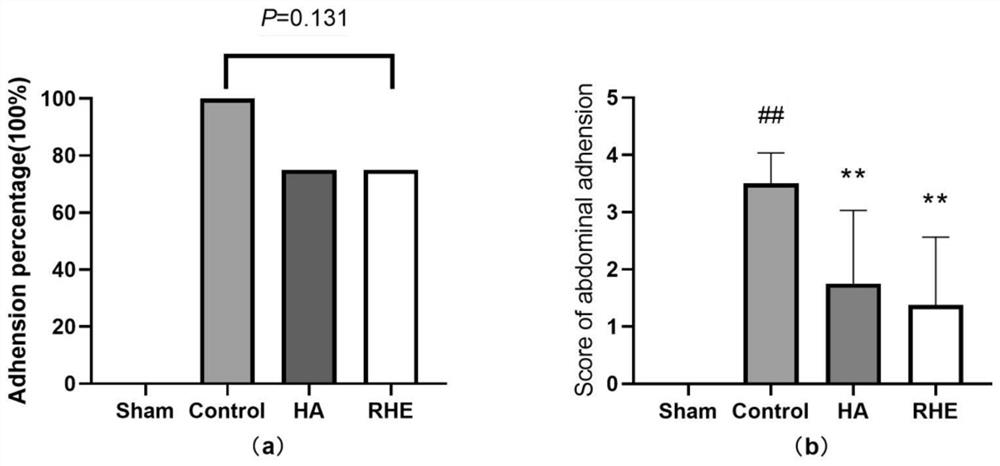
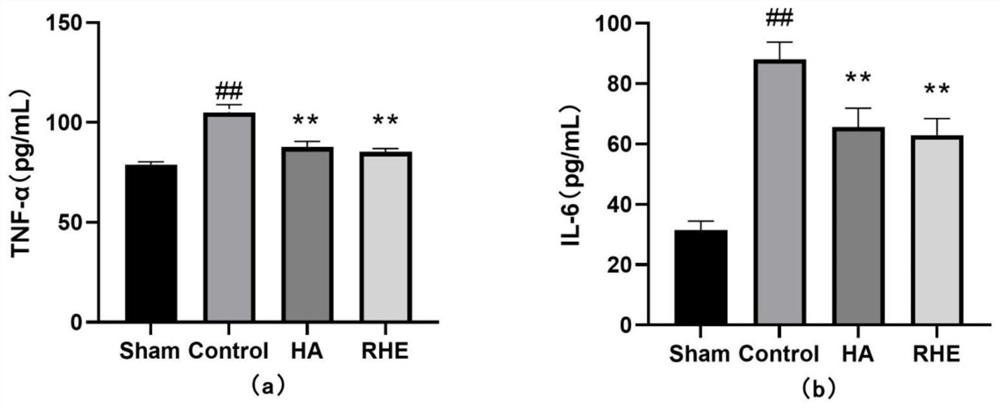
Medicine for treating postoperative abdominal adhesion, and new use of medicine
A drug and intraperitoneal technology, applied in the field of medicine, can solve problems such as poor actual effect of intraperitoneal adhesion, and achieve the effects of reducing economic burden, reducing complications, and reducing average thickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Materials and methods
[0034] 1.1 Animals
[0035] 32 healthy female SD rats, weighing 230-270 g (certificate number SCXK-(Xiang) 2016-0002), were purchased from Hunan Slack Jingda Experimental Animal Co., Ltd., and were fostered in the barrier system of the Department of Experimental Animals, Central South University. . Before the experiment, the rats were adapted to the environment under standard experimental conditions for 7 days, given standard animal feed and water, and the ambient temperature was maintained at 20-26° C., and the relative humidity was 30%-70%. The experimental process followed the principles of humanism to ensure the welfare and ethics of experimental animals. All animal experimental procedures were submitted to the Experimental Animal Welfare Ethics Committee of Central South University for approval and completed in the Department of Experimental Zoology of Central South University.
[0036] 1.2 Drugs and reagents
[0037] Recombinant human...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com