Preparation method of p-hydroxyl biphenol
A technology of hydroxybiphenol and di-tert-butylphenol is applied in the field of preparation of p-hydroxybiphenol, and can solve the problems of low purity of 4,4'-biphenol, many steps, low yield and the like , to achieve the effect of reducing the use of other reagents, good reaction selectivity, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
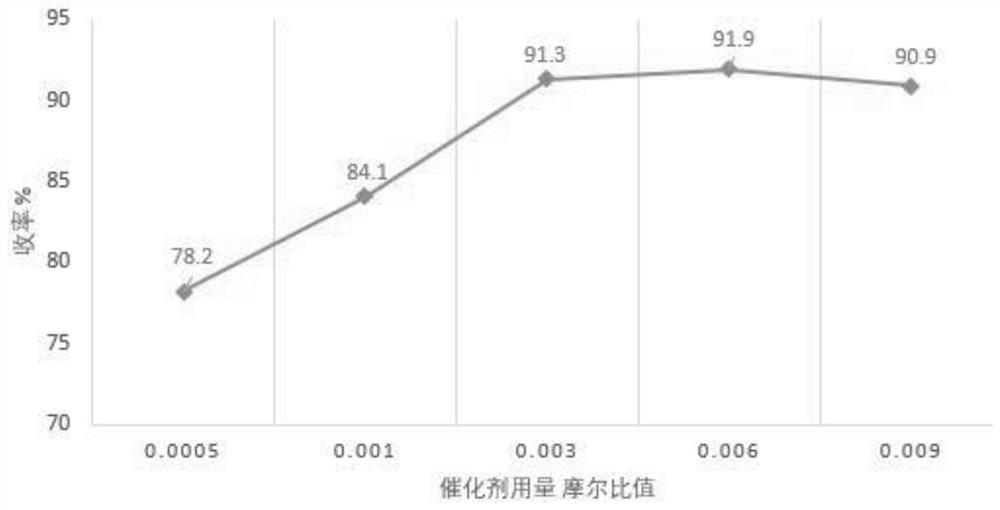
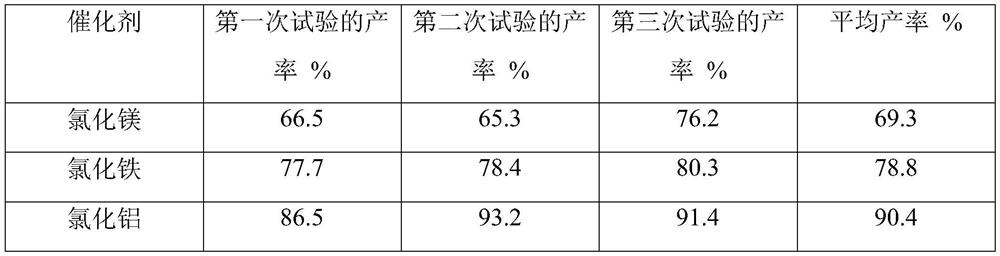
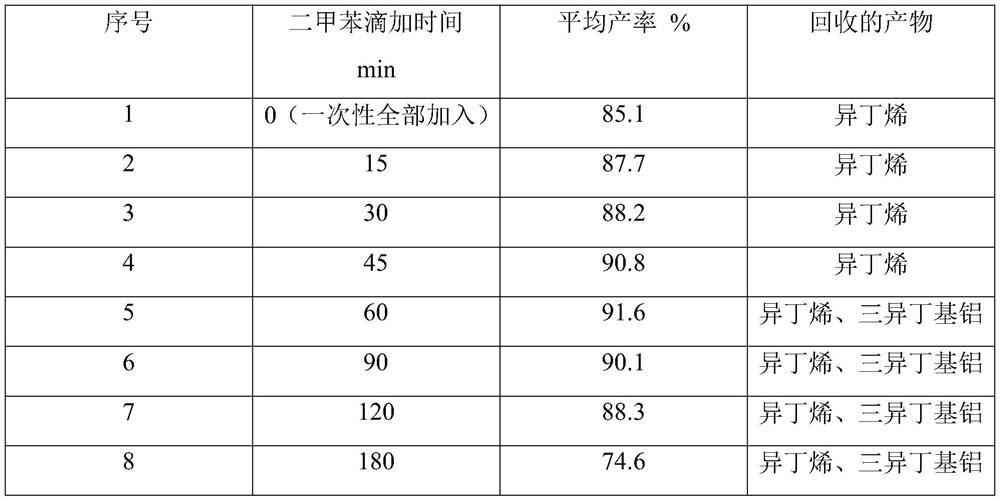
Image
Examples
Embodiment 1
[0042](1) 206.33g of 2,6-di-tert-butylphenol, 106.17g of xylene, and 0.52g of aluminum chloride are respectively added in a 1000ml three-necked flask with a reflux condenser and a packed tower, and clean air is introduced, and the reaction temperature is 50 °C, reacted for 7.5 hours, and the content of oxidation products was 37%. At this time, stop feeding air, switch to nitrogen protection, add 95.86g sodium bisulfite, and carry out reduction reaction at 150° C. for 8 hours, and the content of the reduction product is 73.8%. Cool down to 80°C, filter and transfer to a 1000ml three-neck flask equipped with a reflux condenser and a packed tower for recrystallization and filtration to obtain a white solid.
[0043] (2) Add 0.52g aluminum chloride to the three-necked flask of step (1) under the protection of nitrogen, and add dropwise 106.17g xylene. The dropwise addition is completed within 40 minutes, and the temperature is raised to 150° C. for de-tert-butyl reaction at the sa...
Embodiment 2
[0045] (1) 206.33g of 2,6-di-tert-butylphenol, 212.34g of xylene, and 0.40g of aluminum chloride are respectively added in a 1000ml three-necked flask with a reflux condenser and a packed tower, and clean air is introduced, and the reaction temperature is 50 °C, reacted for 10 hours, and the oxidation product content was 46.5%. At this time, stop feeding air, switch to nitrogen protection, add 93.65g of sodium bisulfite, and carry out reduction reaction at 150°C for 7.5 hours, and the content of the reduction product is 91.5%. Cool down to 80°C, filter and transfer to a 1000ml three-neck flask equipped with a reflux condenser and a packed tower for recrystallization and filtration to obtain a white solid.
[0046] (2) Add 0.40 g of aluminum chloride to the three-necked flask of step (1) under nitrogen protection, and add 212.34 g of xylene dropwise. The addition is completed within 45 minutes, and the temperature is raised to 135° C. for tert-butyl reaction for 6 hours. After...
Embodiment 3
[0048] Add 206.33g of 2,6-di-tert-butylphenol, 318.51g of xylene, and 0.27g of aluminum chloride into a 1000ml three-neck flask equipped with a reflux condenser and a packed tower respectively, pass in clean air, and react at a temperature of 50°C. After 8.5 hours, the oxidation product content was 46%. At this point, stop feeding the air, switch to nitrogen protection, add 104.06g of sodium bisulfite, and carry out reduction reaction at 150° C. for 7 hours, and the content of the reduction product is 91.5%. Cool down to 80°C, filter and transfer to a 1000ml three-neck flask equipped with a reflux condenser and a packed tower for recrystallization and filtration to obtain a white solid.
[0049] (2) Add 0.27g of aluminum chloride to the three-necked flask of step (1) under the protection of nitrogen, and dropwise add 212.34g of dimethylbenzene, dropwise within 50min, and simultaneously raise the temperature to 120°C for tert-butyl reaction for 7 hours, The content of the inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com