Preparation method of imipenem
A technology of imipenem and ammonium chloride, applied in the direction of organic chemistry, can solve the problem of low cost, achieve rapid dissolution, prevent unstable state, and prevent formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
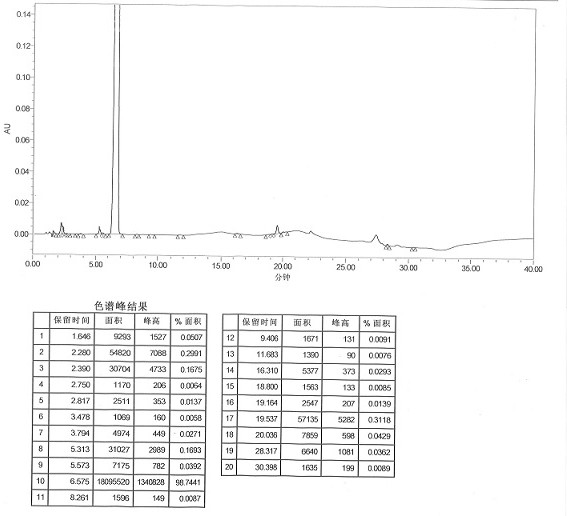
[0047] Measure 800ml of ammonium chloride solution (10%), adjust the pH to 8.5 with ammonia water, measure 750ml of the prepared ammonium chloride-ammonia water inorganic buffer solution and heat to 50°C, add 15g of imipenem crude product, stir to dissolve, Add 4.5 g of activated carbon, and after the temperature drops to 5-10°C, keep stirring at 5-10°C for 30 minutes, and filter to obtain the filtrate. Transfer the filtrate to a crystallization kettle, add 600ml of acetone, keep stirring and crystallizing at 0-5°C for 30 minutes, after the crystals precipitate, add 1400ml of acetone dropwise, after the addition is complete, keep stirring and crystallizing at 0-5°C for 2 hours, then filter with suction. Vacuum-dried to obtain 12.80 g of off-white imipenem monohydrate. Refining yield 85.33%, HPLC detects (see attached figure 2 ): 99.40%, impurity II content 0.0018% (peak elution time 19.7min).
Embodiment 2
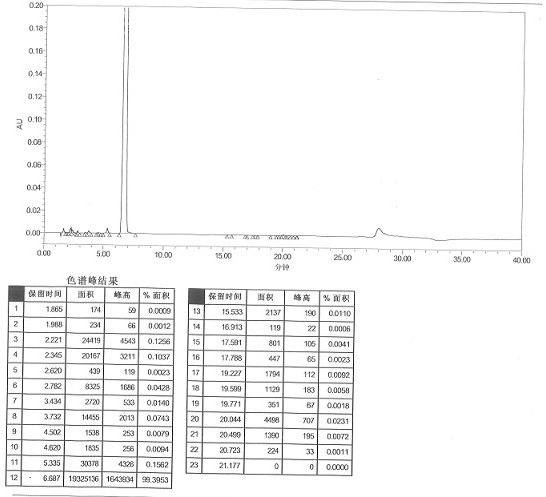
[0049]Measure 800ml of ammonium chloride solution (20%), adjust the pH to 7.1 with ammonia water, measure 600ml of the prepared ammonium chloride-ammonia water inorganic buffer solution and heat to 50°C, add 15g of imipenem crude product, stir to dissolve, Add 4.5 g of activated carbon, and after the temperature drops to 5-10°C, keep stirring at 5-10°C for 30 minutes, and filter to obtain the filtrate. Transfer the filtrate to a crystallization kettle, add 480ml of acetone, keep stirring and crystallizing at 0-5°C for 30 minutes, after the crystals precipitate, add 1200ml of acetone dropwise, after the addition is complete, keep stirring and crystallizing at 0-5°C for 2 hours, then filter with suction. After vacuum drying, 12.72 g of off-white imipenem monohydrate was obtained. Refining yield 84.80%, HPLC detects (referring to attached figure 2 ): 99.39%, impurity II content 0.0021%.
Embodiment 3
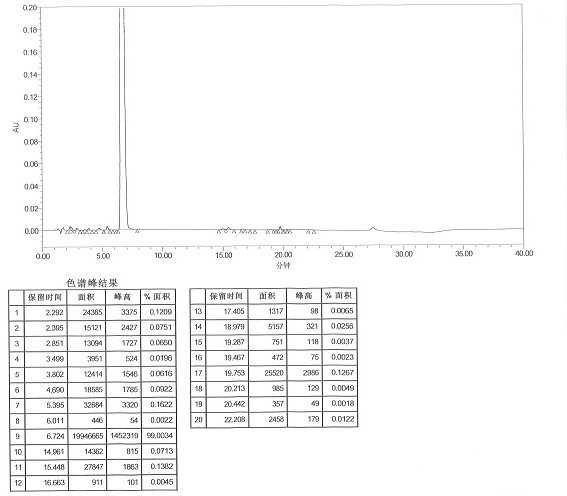
[0051] Measure 800ml of ammonium chloride solution (1%), adjust the pH to 9 with ammonia water, measure 600ml of the prepared ammonium chloride-ammonia water inorganic buffer solution and heat to 50°C, add 7.5g of imipenem crude product, stir to dissolve , add 2.3g of activated carbon, after the temperature drops to 5-10°C, keep stirring at 5-10°C for 30min, and filter to obtain the filtrate. Transfer the filtrate to a crystallization kettle, add 480ml of acetone, keep stirring and crystallizing at 0-5°C for 30 minutes, after the crystals precipitate, add 1200ml of acetone dropwise, after the addition is complete, keep stirring and crystallizing at 0-5°C for 2 hours, then filter with suction. Vacuum-dried to obtain 6.38 g of off-white imipenem monohydrate. Refining yield 85.06%, HPLC detects (referring to attached figure 2 ): 99.38%, impurity II content 0.0020%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com