Waterborne polyurethane, and preparation method and application thereof
A water-based polyurethane, reaction technology, applied in the directions of polyurea/polyurethane coatings, polyurea/polyurethane adhesives, sulfide preparation, etc. Effects of Excessive Stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
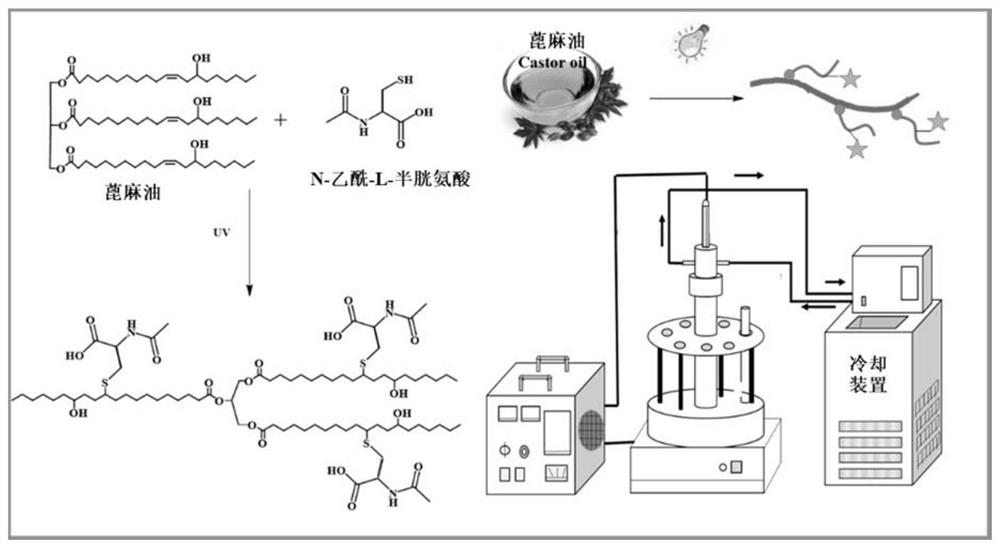
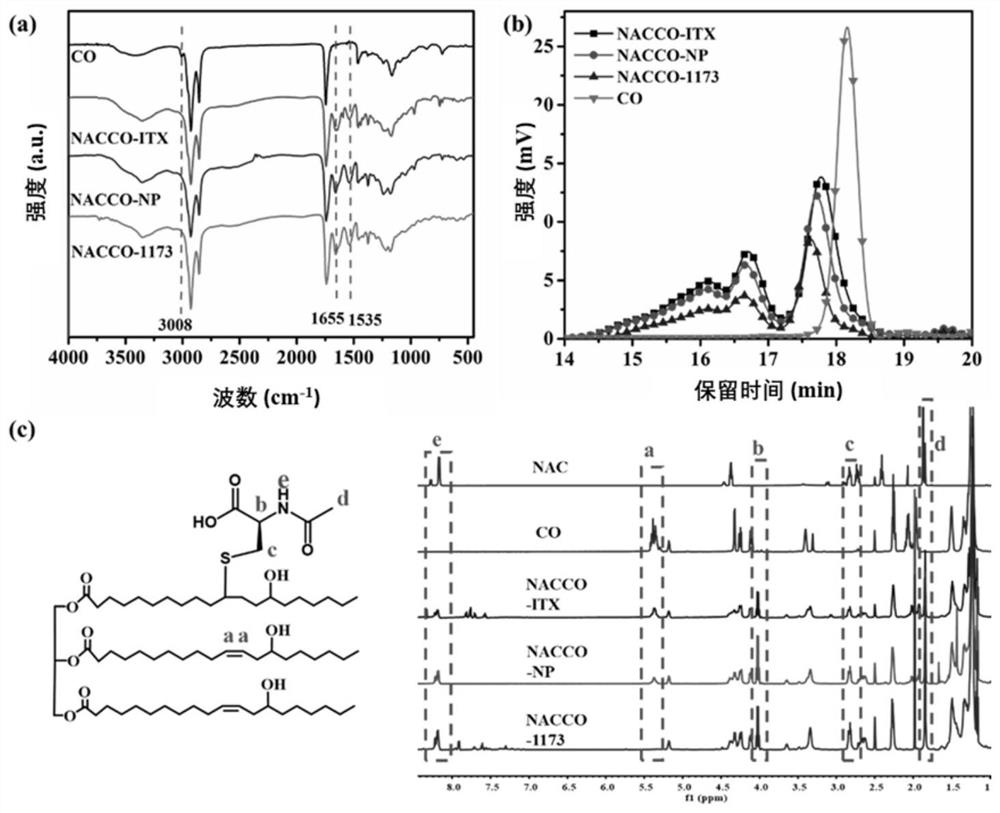
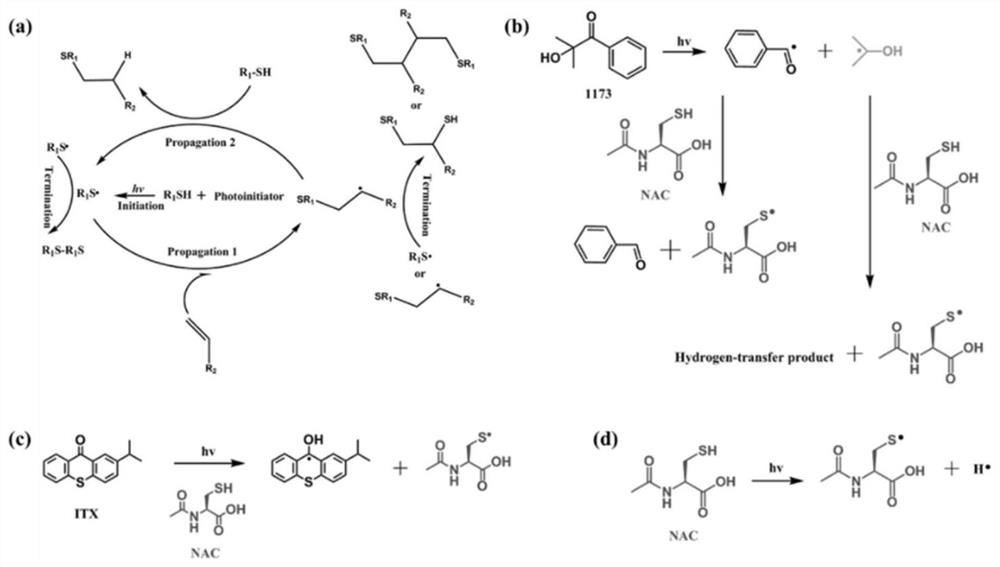
[0096]This example provides a series of chain extenders NACCO, which are prepared by the click reaction of castor oil (CO) and N-acetyl-L-cysteine (NAC). The specific process is as follows.
[0097]First, an appropriate ratio of NAC, CO and photoinitiator (4wt.%) were dissolved in absolute ethanol and transferred to a quartz tube. Under the ultraviolet irradiation of 350W power, the reaction time is certain (reaction equipment such asfigure 1 ). After that, the product was extracted with ethyl acetate and dried with anhydrous magnesium sulfate. The product was filtered and rotary evaporated to remove ethyl acetate, and then dried overnight under vacuum at 45°C to obtain the chain extender NACCO.
[0098]Specifically, two photoinitiators (1173, ITX), 6 different reaction times (5min, 10min, 15min, 20min, 25min and 30min) and 6 different sulfhydryl / double bond molar ratios (1:1, 2: 1, 3:1, 4:1, 5:1 and 6:1), the obtained chain extenders were named NACCO-p, NACCO-T and NACCO-m, where p, T ...
Embodiment 2~9
[0122]This example provides a series of chain extenders, which are prepared by the click reaction of castor oil and N-acetyl-L-cysteine derivatives. The structural parameters of N-acetyl-L-cysteine derivatives are shown in Table 4 Shown.
[0123]Table 4 The structure of N-acetyl-L-cysteine derivatives
[0124]
[0125]Castor oil and N-acetyl-L-cysteine derivatives perform a click reaction
[0126]The N-acetyl-L-cysteine derivatives (derivatives 1-8), CO and photoinitiator (4wt.%) were dissolved in absolute ethanol and transferred to a quartz tube. Under the UV irradiation of 350W power, the reaction time is 15min. After that, the product was extracted with ethyl acetate and dried with anhydrous magnesium sulfate. The product was filtered and rotary evaporated to remove ethyl acetate, and then dried overnight at 45°C in a vacuum environment to obtain a chain extender, which was recorded as Examples 2-9 in turn. Table 5 shows its performance characteristics.
[0127]Table 5 The acid value,...
Embodiment 10
[0130]This example provides a series of waterborne polyurethane emulsions, using NACCO (NACCO-4:1) provided in Example 1 as a chain extender, and prepared by the following process.
[0131]Such asFigure 6 , Polyol (PPG, PCDL, CO, castor oil derivatives) and isophorone diisocyanate (IPDI) were added to a two-neck flask equipped with mechanical stirring, and stirred and mixed at 78°C for 10 min. Then, dibutyltin laurate (DBTDL) (1% mass fraction of polyol) was added to the mixture, and then reacted for 20 minutes. Subsequently, the NACCO chain extender was dissolved in methyl ethyl ketone (MEK) and added dropwise to the mixture with stirring. The dropwise addition was completed within 60 minutes. The amount of reactant is shown in Table 6. After the reaction, 5-10 mL of MEK was poured into the mixture to reduce the viscosity of the system. Then, when the temperature is cooled to room temperature, the system is neutralized with TEA and stirred for about 30 minutes. Finally, the mixture wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com