Phenanthroimidazole compound substituted by diphenylphosphine oxide, preparation method and application thereof
A technology of diphenylphosphine and phenanthroimidazole, which is applied in the application field of organic light-emitting materials and optoelectronic devices, can solve the problems of not being able to have both good carrier transport balance and high blue light color purity, and achieve good current carrier The effect of sub-transport balance ability, high blue light color purity, and high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
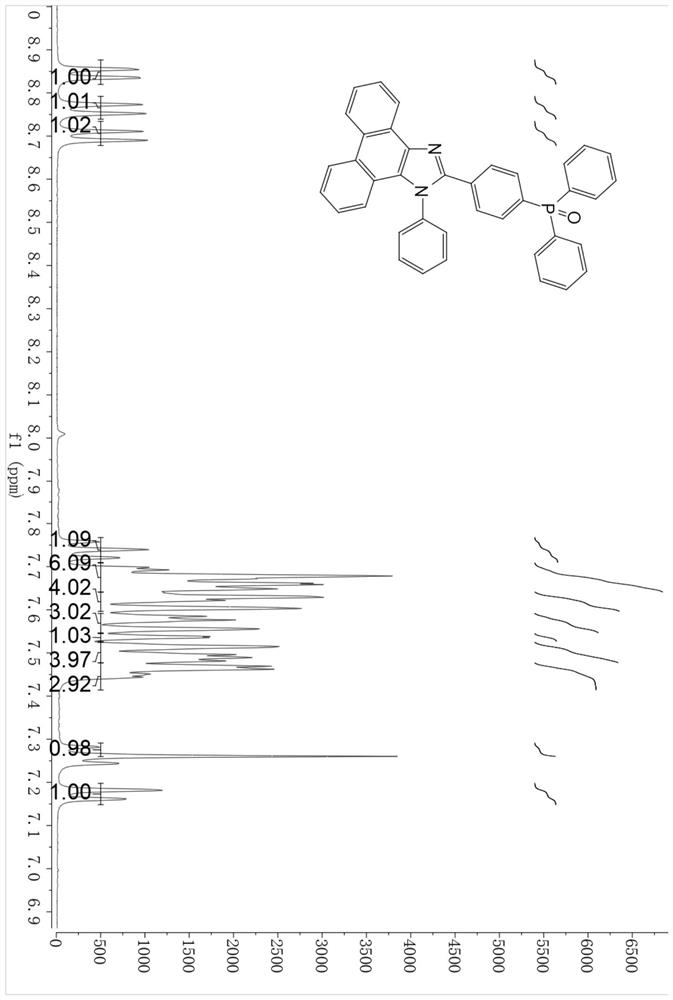
[0045] A diphenylphosphine-substituted phenanthroimidazole compound has a molecular structure shown in formula (I):
[0046] Wherein R is a hydrogen atom or diphenylphosphine, when R is a hydrogen atom, it is named M1, and its molecular structure is:
[0047] When R is diphenylphosphine, it is named as M2, and its molecular structure is:
[0048] The preparation method of the phenanthroimidazoles substituted by the above-mentioned diphenylphosphine, comprising the steps of:
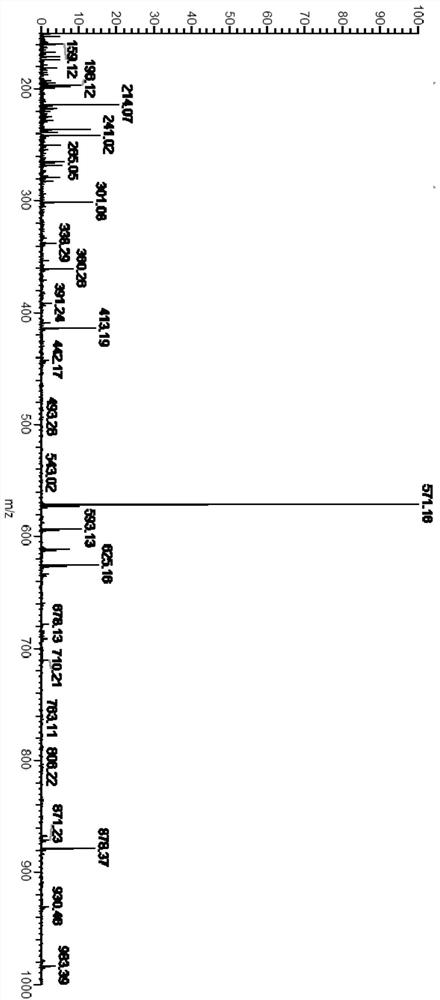
[0049] S1. Add 4-bromobenzaldehyde (1.86g, 10mmol), p-bromoaniline (1.70g, 10mmol), 9,10-phenanthrenequinone (2.08g, 10mmol), ammonium acetate (4.62g, 60mmol) into 100ml of In a two-necked flask (4-bromobenzaldehyde, bis-p-bromoaniline and 9,10-phenanthrenequinone in a molar ratio of 1:1:1), add 60ml of glacial acetic acid to obtain a dark brown suspension. After the mixture was stirred at 120° C. for 2 hours, the color of the solution changed from dark brown to black, and the reaction mixture was...
Embodiment 2
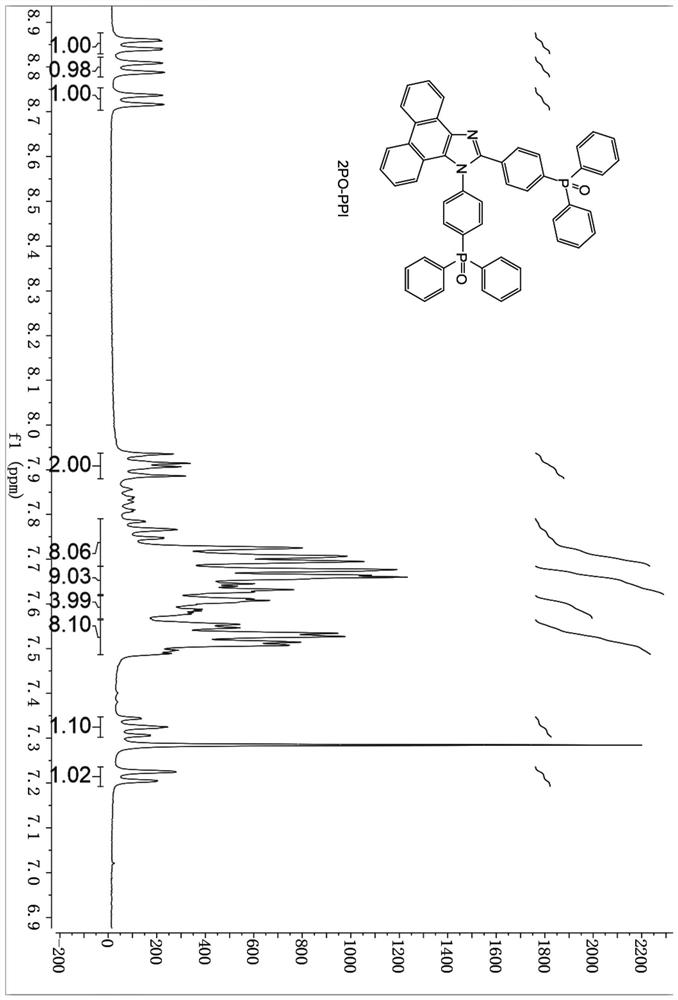
[0058] A diphenylphosphine-substituted phenanthroimidazole compound has a molecular structure shown in formula (I):
[0059] Wherein R is a hydrogen atom or diphenylphosphine, when R is a hydrogen atom, it is named M1, and its molecular structure is:
[0060] When R is diphenylphosphine, it is named as M2, and its molecular structure is:
[0061] The preparation method and separation steps of the above-mentioned diphenylphosphine-substituted phenanthrene imidazole compounds are the same as in Example 1, the difference is that the 4-bromobenzaldehyde, bis-p-bromoaniline and 9,10-phenanthrenequinone described in step S1 The molar ratio is replaced by 1:1:2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com