Feruloyl amide derivative as well as medical application and crystal structure thereof
A technology of ferulamide and its derivatives, which is applied in the field of new compounds and can solve problems such as the reduction of NA inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of (E)-N-(4-tert-butyl-5-nitrothiazol-2-yl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide
[0057]
[0058] 0.23g (1.2mmol) ferulic acid, 0.16g (1.2mmol) 1-hydroxybenzotriazole, 0.23g (1.2mmol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Dissolve hydrochloride in 10 mL of N,N dimethylformamide and stir for 30 min, add 0.20 g (1 mmol) of 2-amino-4-tert-butyl-5-nitrothiazole, heat up to 115°C and react for 16 h (monitored by TLC). The reaction solution was cooled to room temperature, poured into ice water, and the precipitated solid was filtered by suction, washed with saturated sodium bicarbonate solution, dilute hydrochloric acid, and water successively, and the crude product was recrystallized from ethanol to obtain red powder (E)-N-(4-tert-butyl -5-nitrothiazol-2-yl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide, yield 36.8%, m.p.262~263°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 1.50(s, 9H, 3×CH 3 ), 3.88 (s, 3H, CH 3 ), 6.82 (d, J=15.6Hz, 1H, =CH), 6.91 (d, J=8.0Hz, ...
Embodiment 2
[0060]Preparation of (E)-N-(4-tert-butyl-5-(imidazol-1-yl)thiazol-2-yl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide
[0061]
[0062] According to the method of Example 1, 0.23g (1.2mmol) ferulic acid and 0.20g (1mmol) 2-amino-4-tert-butyl-5-imidazolhiazole were reacted at 115°C for 10h (monitored by TLC), and the crude product was washed with ethanol Recrystallized to give light yellow powder (E)-N-(4-tert-butyl-5-(imidazol-1-yl)thiazol-2-yl)-3-(4-hydroxy-3-methoxyphenyl) Acrylamide, yield 38.2%, m.p.244~246°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 1.15(s, 9H, 3×CH 3 ), 3.85(s, 3H, OCH 3 ), 6.77 (d, J=15.6Hz, 1H, =CH), 6.88 (d, J=7.6Hz, 1H, C 6 h 3 ), 7.12~7.14(m, 2H, imidazole ring-H+C 6 h 3 ), 7.21(s, 1H, C 6 h 3 ) 7.47 (s, 1H, imidazole ring-H), 7.67 (d, J=15.6Hz, 1H, =CH), 7.94 (s, 1H, imidazole ring-H), 9.71 (s, 1H, OH), 12.40 (s, 1H, NH).
Embodiment 3
[0064] (E)-N-(4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl)-3-(4-hydroxy-3-methoxyphenyl ) Preparation of acrylamide
[0065]
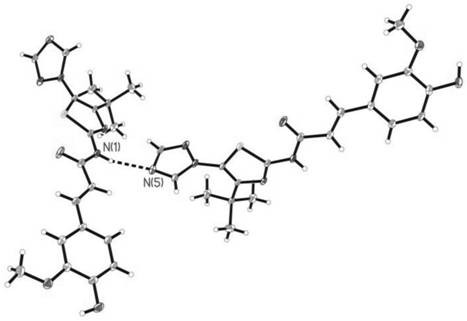
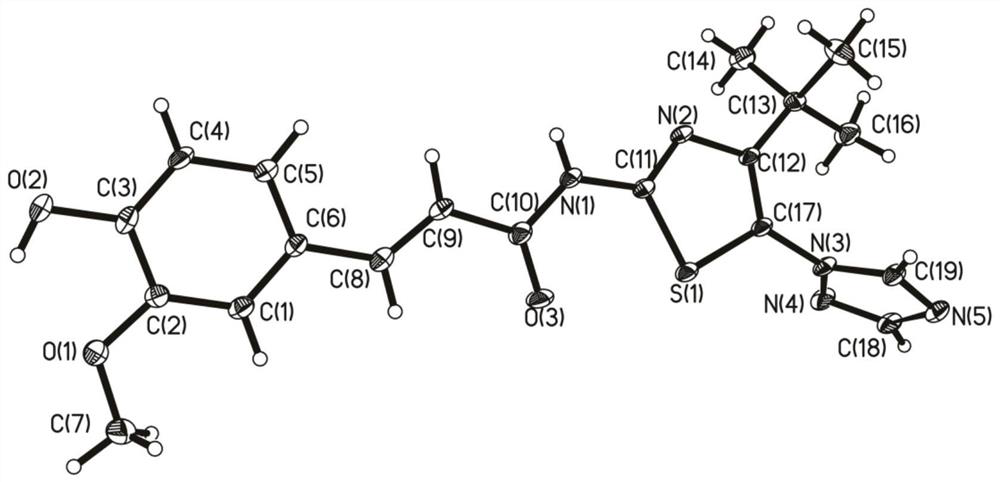
[0066] According to the method of Example 1, 0.23g (1.2mmol) ferulic acid was reacted with 0.22g (1mmol) 2-amino-4-tert-butyl-5-(1,2,4-triazole)thiazole at 115°C 10h (monitored by TLC), the crude product was recrystallized from ethanol to obtain beige powder (E)-N-(4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazol-2-yl) -3-(4-Hydroxy-3-methoxyphenyl)acrylamide, yield 41.5%, m.p.196~198°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 1.14(s, 9H, 3×CH 3 ), 3.85(s, 3H, OCH 3 ), 6.78 (d, J=15.6Hz, 1H, =CH), 6.88 (d, J=8.0Hz, 1H, C 6 h 3 ), 7.14 (d, J=8.0Hz, 1H, C 6 h 3 ), 7.22(s, 1H, C 6 h 3 ), 7.69 (d, J=15.6Hz, 1H, =CH), 8.28 (s, 1H, triazole ring-H), 8.98 (s, 1H, triazole ring-H), 9.74 (s, 1H, OH ), 12.53 (s, 1H, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com