Method for detecting in-vitro efficacy of mycoplasma hyopneumoniae inactivated vaccine based on ELISA method
A technology of Mycoplasma hyopneumoniae and inactivated vaccine is applied in the field of testing the relative efficacy of Mycoplasma hyopneumoniae inactivated vaccine (DJ166 strain) in vitro, which can solve the problems of long time for animal testing, high testing cost, affecting the repeatability of testing, etc. Cost and labor intensity of animal testing, good sensitivity and specificity, good specificity and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] This example is used to prepare Mycoplasma hyopneumoniae inactivated vaccine (DJ166 strain) and its reference vaccine (batch number ZYTCK-1801).
[0059] Mycoplasma hyopneumoniae inactivated vaccine (DJ166 strain) reference vaccine (batch number: ZYTCK-1801) is produced by the applicant in strict accordance with the current "Chinese Veterinary Pharmacopoeia" standard, and the inactivated mycoplasma hyopneumoniae vaccine (DJ166 strain) to be inspected (batch number: ZYT -191, ZYT-192) are also produced by the applicant in strict accordance with the current "Chinese Veterinary Pharmacopoeia" standards.
[0060] The reference vaccine ZYTCK-1801 and the inactivated vaccine of Mycoplasma hyopneumoniae (DJ166 strain) to be tested (batch number: ZYT-191, ZYT-192) were inoculated with the appropriate culture medium with Mycoplasma hyopneumoniae DJ-166 strain, harvested, concentrated and purified Afterwards, the antigen is prepared by thimerosal inactivation, multiple antigen co...
Embodiment 2
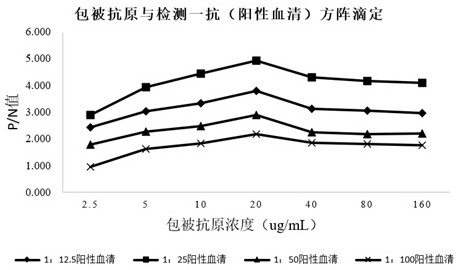
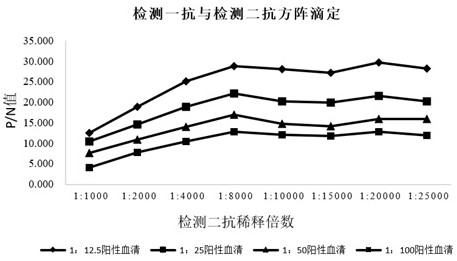
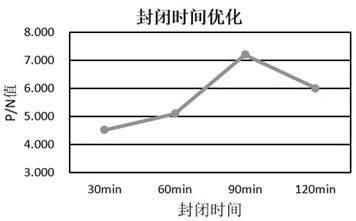
[0072] The preparation method of mycoplasma competitive coating antigen in this example includes the following steps: the concentrated and purified bacterial liquid of Mycoplasma hyopneumoniae DJ-166 strain is inactivated, centrifuged, and combined with TritonX-100 and KI solution. It is used to test the relative potency of Mycoplasma hyopneumoniae inactivated vaccine (DJ-166 strain) in vitro by indirect competitive inhibition ELISA test.
[0073] Coating antigen quality control
[0074] Properties: After dissolving and shaking well, it becomes a milky white liquid;
[0075] Sterility test: should grow sterile;
[0076] Potency determination: carry out agar diffusion test with positive serum of Mycoplasma hyopneumoniae, the agar expansion titer should not be lower than 1:2;
[0077] Determination of protein concentration: use a commercial BCA kit for detection, and the protein concentration should not be lower than 1.0mg / ml;
[0078] Specificity: There should be a clear whi...
Embodiment 3
[0081] The preparation method of the positive serum in this example includes the following steps: immunize healthy rabbits with the inactivated Mycoplasma hyopneumoniae vaccine, and inoculate once with the same dose and route 2 weeks after the immunization, when the antibody titer is not lower than 1:16 It is prepared by aseptic blood collection, serum separation, filter sterilization, inactivation and purification, and freeze-drying in vacuum. Positive serum for indirect competitive inhibition ELISA test for inactivated Mycoplasma hyopneumoniae inactivated vaccine (DJ-166 strain) in vitro, which can be freeze-dried in batches.
[0082] Positive serum freeze-dried quality control standard
[0083] Properties: It should be a white spongy loose mass;
[0084] Sterility test: should grow sterile;
[0085] Titer determination: carry out agar diffusion test with Mycoplasma hyopneumoniae antigen, the agar expansion titer should not be lower than 1:2;
[0086] Specificity: There s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com