Applications of apatinib in preparation of drugs for preventing and treating radiation induced brain injury
A technology of radiation brain injury and apatinib, which is applied in the field of medicine, can solve the problems of high price, high risk and easy recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
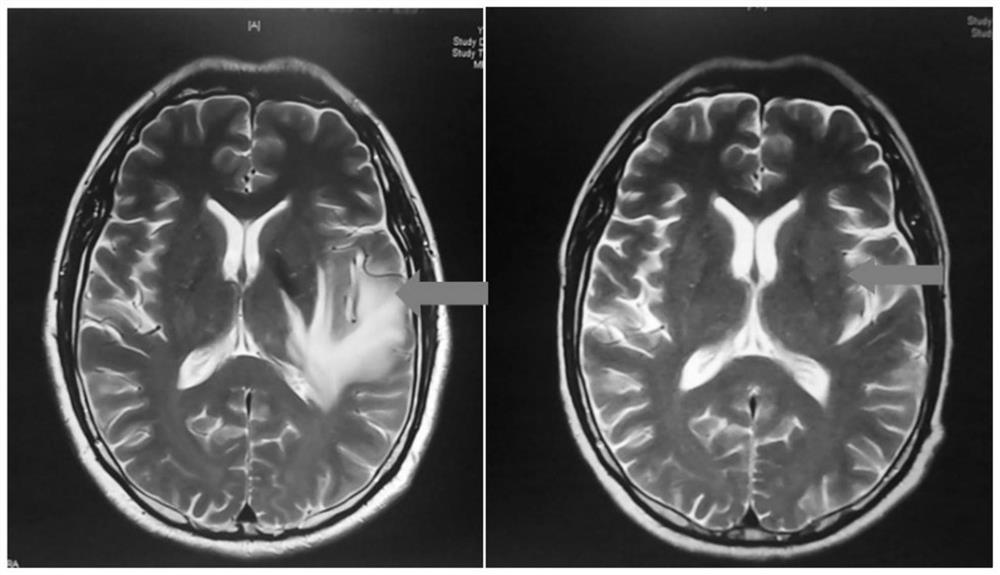
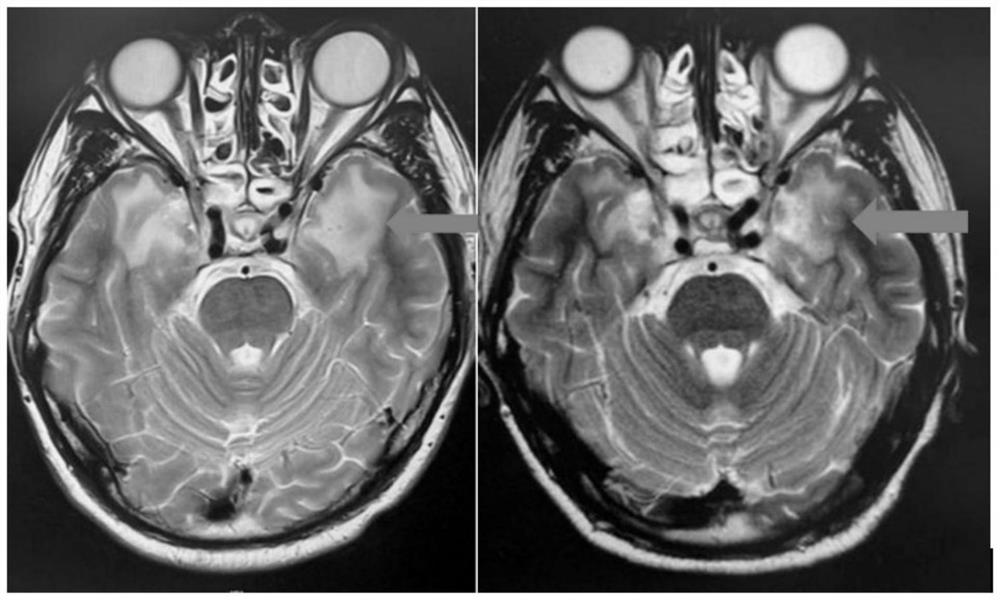
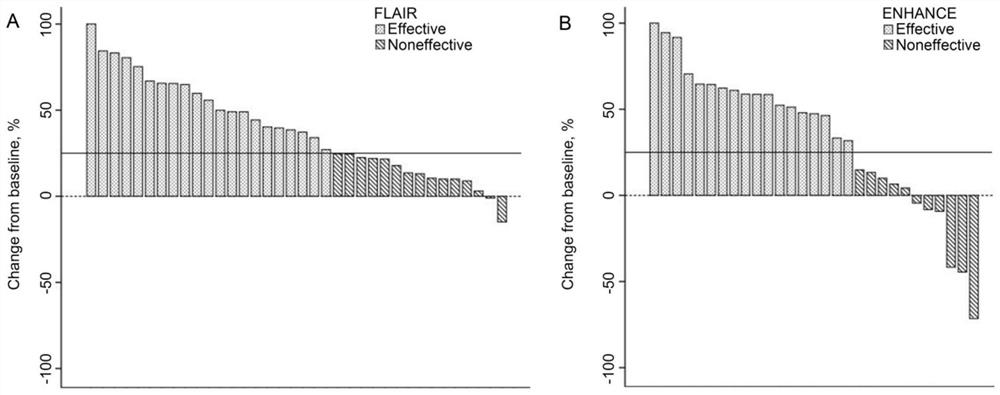
[0017] According to the international paradigm of exploring new indications of drugs, on the basis of completing the exploratory observation of individual clinical cases, a phase II clinical study of apatinib mesylate on the treatment of radiation encephalopathy was conducted to explore its efficacy and conduct safety evaluation.
[0018] Subjects: Patients with radiation encephalopathy
[0019] Inclusion criteria: 36 cases were enrolled, aged 18 to 80 years, ① Radiation encephalopathy was diagnosed by cranial magnetic resonance (MRI) or pathologically confirmed; ② Radiation brain injury was first diagnosed; ③ Tumor recurrence, invasion, metastasis, and surgery were excluded Indications (see subject selection below for details).
[0020] Medication method: Apatinib 250mg qd. The total course of treatment is 4 weeks.
[0021] Observation indicators: before the start of treatment and after 4 weeks of treatment, neurological physical examination assessment, head MRI examination...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com