Aminocomprolizine derivatives and their applications
A technology of amino and drugs, which is applied in the synthesis of anticancer drug compounds, aminocompritine derivatives and their synthesis, and preparation fields, which can solve the problems of reduced human tolerance, increased toxicity, and unsuitable clinical use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
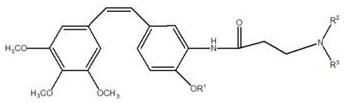
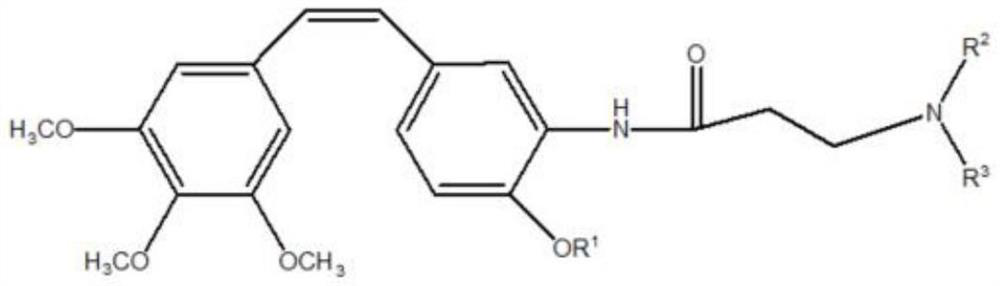
[0051] (Z)-1-(3,4,5-trimethoxyphenyl)-2-(3-(bis(2-n-propyl)amino)propionamide)-4-ethoxyphenyl)ethene synthesis
[0052]
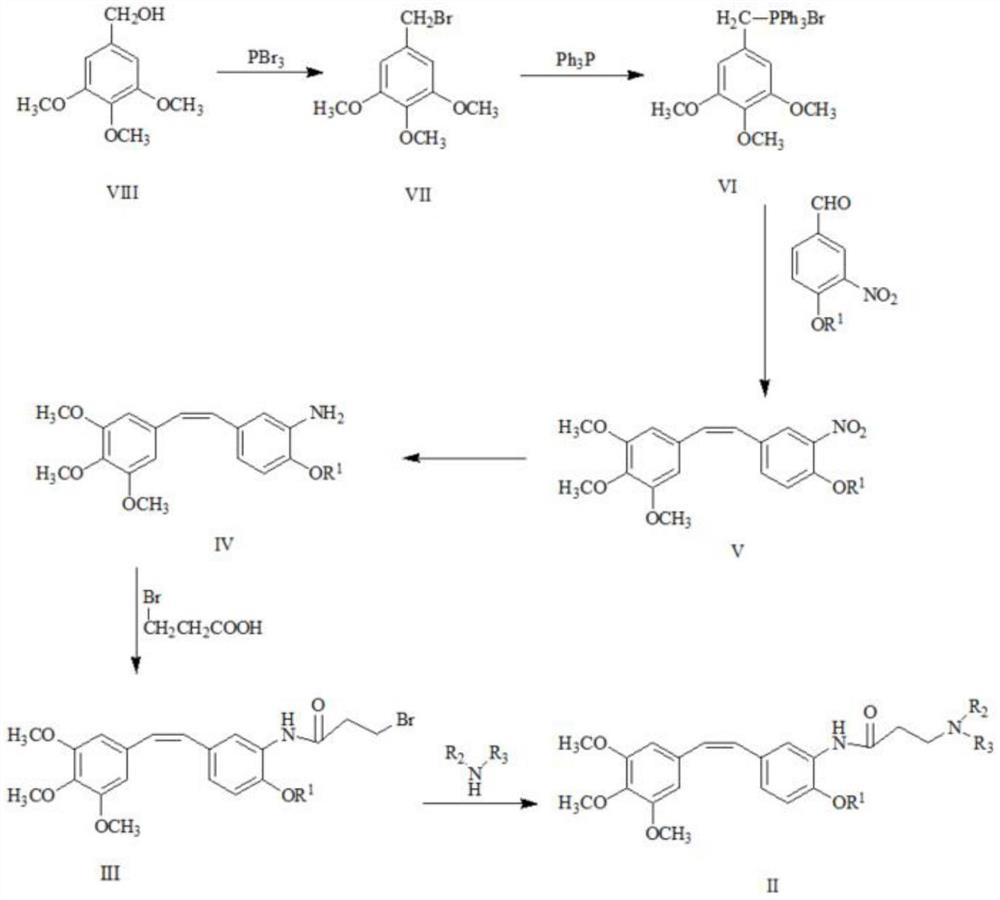
[0053] Step 1: Synthesis of trimethoxybenzyltriphenylphosphine bromide
[0054] Dissolve 320 g of 3,4,5-trimethoxybenzyl alcohol in 2L of toluene, stir to dissolve, and then cool down to -5 to 0 °C, add 100 ml of phosphorus bromide dropwise to a constant pressure dropping funnel, and keep the reaction temperature at -5 to 0 °C. After the dropwise addition was completed, the reaction was continued at low temperature for 2 hours, and then returned to room temperature, and the reaction was performed overnight.
[0055] Add 1.4 L of purified water to quench the reaction, stir for 30 min, and let stand for separation. The upper organic phase was washed with saturated sodium bicarbonate solution to pH 7.5-8, dried over anhydrous magnesium sulfate and filtered to obtain a toluene solution of trimethoxybenzyl bromide.
[0056] Add 0.56kg of triphenylphosphoru...
Embodiment 2
[0066] (Z)-1-(3,4,5-Trimethoxyphenyl)-2-((3-(bis(2-n-propyl)amino)propionamide)-4-ethoxyphenyl)ethene Preparation of hydrochloride
[0067]1 g of (Z)-1-(3,4,5-trimethoxyphenyl)-2-((3-(bis(2-n-propyl)amino)propionamide)-4-ethoxyphenyl ) Ethylene was dissolved in 10 ml of methanol, 0.2 ml of hydrochloric acid was added, heated to 35 °C and stirred for 1 h. After cooling, it was concentrated to dryness, 10 ml of water was added, stirred for 10 min, and then allowed to stand overnight. Suction filtration, the obtained white filter cake is (Z)-1-(3,4,5-trimethoxyphenyl)-2-((3-(bis(2-n-propyl)amino)propane) after freeze-drying amide)-4-ethoxyphenyl)ethylene hydrochloride.
Embodiment 3
[0069] (Z)-1-(3,4,5-Trimethoxyphenyl)-2-((3-(bis(2-n-propyl)amino)propionamide)-4-methoxyphenyl)ethene preparation
[0070]
[0071] Step 1: Synthesis of trimethoxybenzyltriphenylphosphine bromide
[0072] Dissolve 320 g of 3,4,5-trimethoxybenzyl alcohol in 2L of toluene, stir to dissolve, and then cool down to -5 to 0 °C, add 100 ml of phosphorus bromide dropwise to a constant pressure dropping funnel, and keep the reaction temperature at -5 to 0 °C. After the dropwise addition was completed, the reaction was continued at low temperature for 2 hours, and then returned to room temperature, and the reaction was performed overnight.
[0073] Add 1.4 L of purified water to quench the reaction, stir for 30 min, and let stand for separation. The upper organic phase was washed with saturated sodium bicarbonate solution to pH 7.5-8, dried over anhydrous magnesium sulfate and filtered to obtain a toluene solution of trimethoxybenzyl bromide.
[0074] Add 0.56kg of triphenylphosp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com