Preparation method of 2-n-butyl-4-chloro-5-formyl imidazole
A technology of formyl imidazole and methyl imidazole, which is applied in the field of preparation of 2-n-butyl-4-chloro-5-formyl imidazole, can solve the problems of difficult mixing, unsatisfactory flow state, low reaction rate and the like, and achieves The effect of improving flow state, fast and uniform mixing reaction, and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Such as Figure 1 to Figure 7 Shown, a kind of preparation method of 2-n-butyl-4-chloro-5-formyl imidazole comprises the following steps:
[0032] S1, cyclization of valeraldehyde and methylglyoxal to obtain 2-butyl-5-methylimidazole;
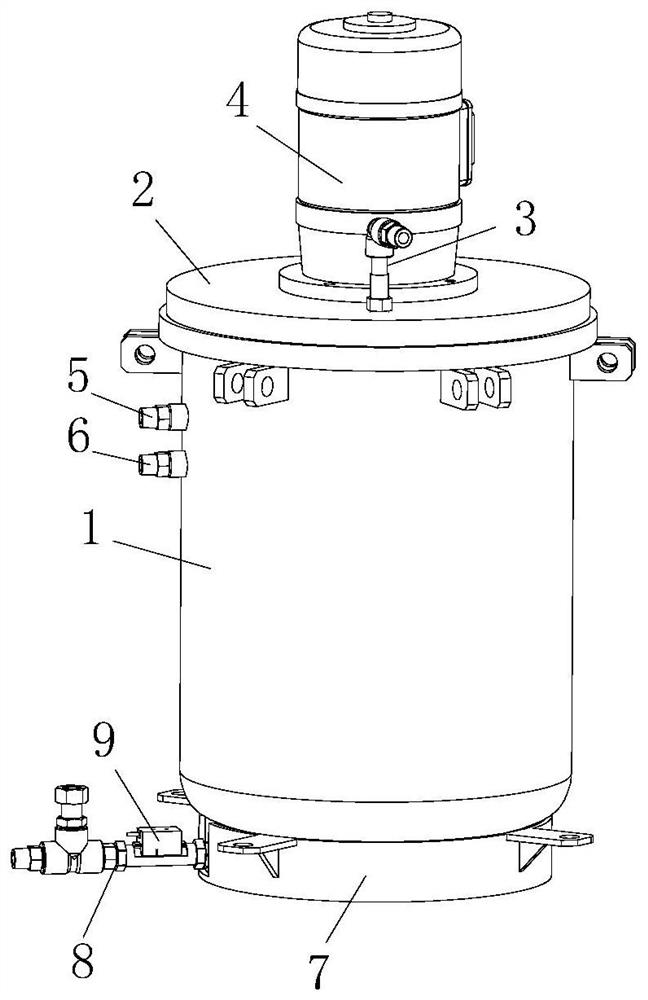
[0033] S2. Add the 2-butyl-5 methylimidazole obtained in step S1 to the reaction kettle through the addition pipe, add the chlorination reagent to the reaction kettle through the second feed pipe 6, and add water and catalyst, and stir evenly. Synthesis of 2-butyl-4-chloro-5-methylimidazole under the action of chlorination reagent, the reaction temperature is 35-40°C, and the reaction time is 6-10 hours;
[0034] S3. Add sodium persulfate in batches to the 2-butyl-4-chloro-5-methylimidazole obtained in step S2 through the first feed pipe 5, heat up to 50-55°C and react for 3-5 hours to obtain 2-n-Butyl-4-chloro-5-formyl imidazole;
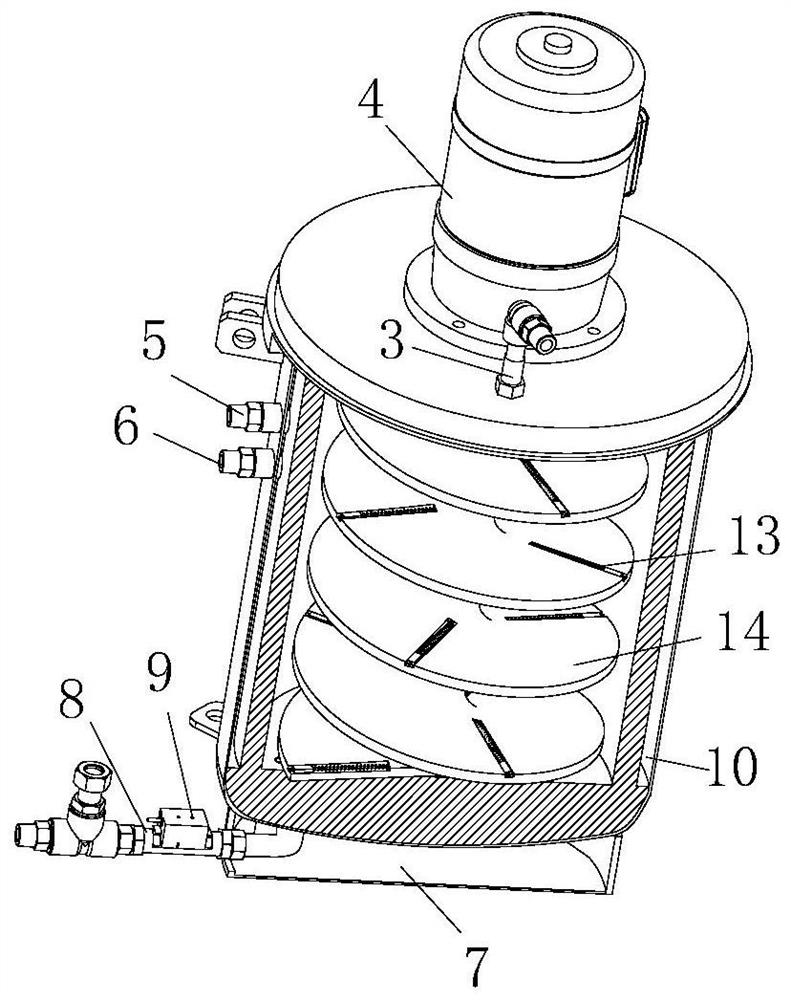
[0035] Wherein, the reaction kettle includes a kettle body 1, the upper end of the kettle body 1 is pro...
Embodiment approach
[0037] As an embodiment of the present invention, the spiral stirring plate 14 is provided with a plurality of radially arranged chute 15 at intervals, and the inner wall of the chute 15 is fixedly provided with a second rod 24 extending outward, The second rod 24 is covered with a second magnetic block 26 that can slide along its extension direction, and the second magnetic block 26 is connected to the inner wall of the chute 15 through a second spring 25, and the second spring 25 Set outside the second pole 24. Through the setting of the chute 15, a part of the material flowing up along the upper surface of the spiral stirring plate 14 can be intercepted, and part of the material in the chute 15 moves outward along the chute 15 to participate in the mixing between the materials on the periphery of the spiral stirring plate 14 At the same time, part of the material stays in the chute 15 and mixes with the subsequent intercepted material, thus improving the effect and efficien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com