Novel coronavirus RBD fusion protein subunit vaccine as well as preparation method and application thereof
A technology of subunit vaccine and coronavirus, which is applied in the field of novel coronavirus RBD fusion protein subunit vaccine and its preparation, can solve problems such as unseen results reports, achieve simple process route, high recovery rate, ensure stability and immunity original effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of a novel coronavirus RBD fusion protein subunit vaccine:
[0043] 1. Construction of recombinant plasmids
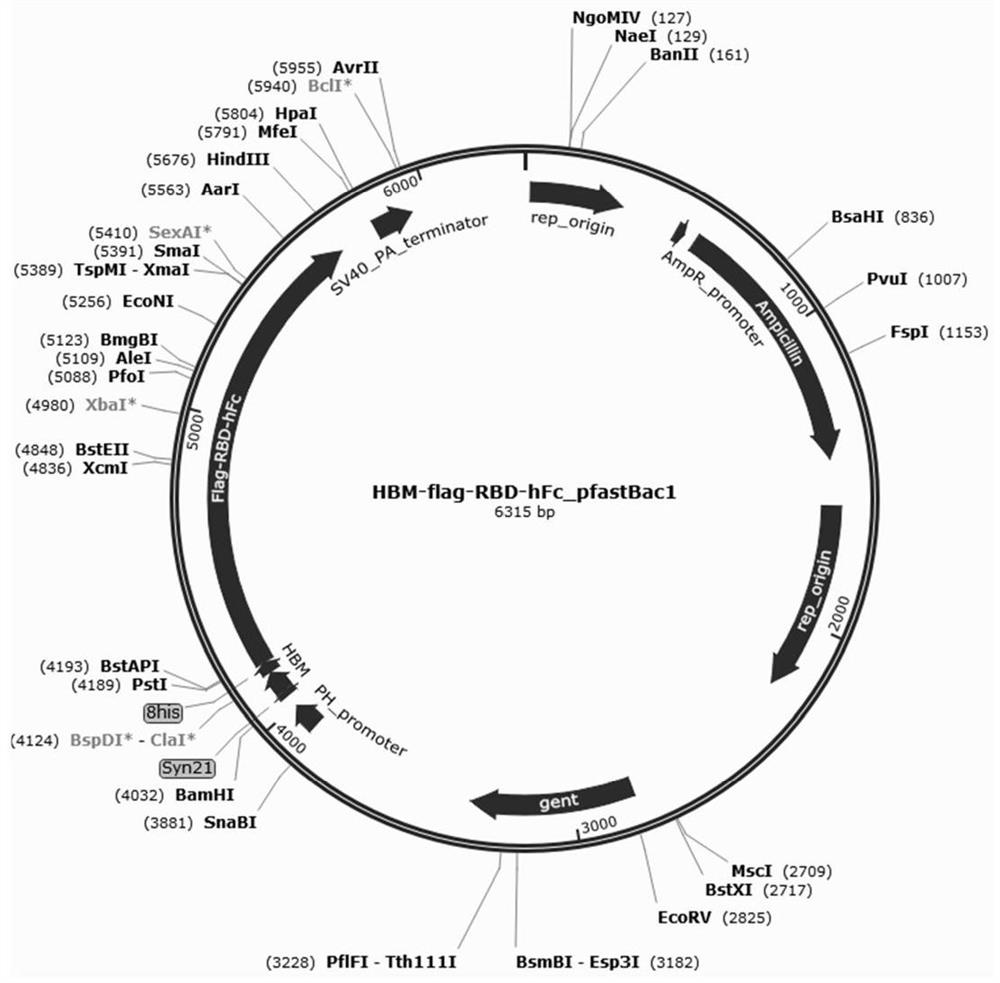
[0044] Select the C-terminal domain RBD, HBM (secretion signal peptide), flag (immune enhancement peptide) and hFc (immune enhancement peptide region) fragments in the S protein sequence of the new coronavirus (COVID-19), and fuse them in series , designed and synthesized on the pfastBac1 vector using codon optimization system, the structure diagram of the constructed recombinant plasmid HBM-flag-RBD-hFc_pfastBac1 is as follows figure 1 shown;
[0045] 2. Construction of recombinant bacmid
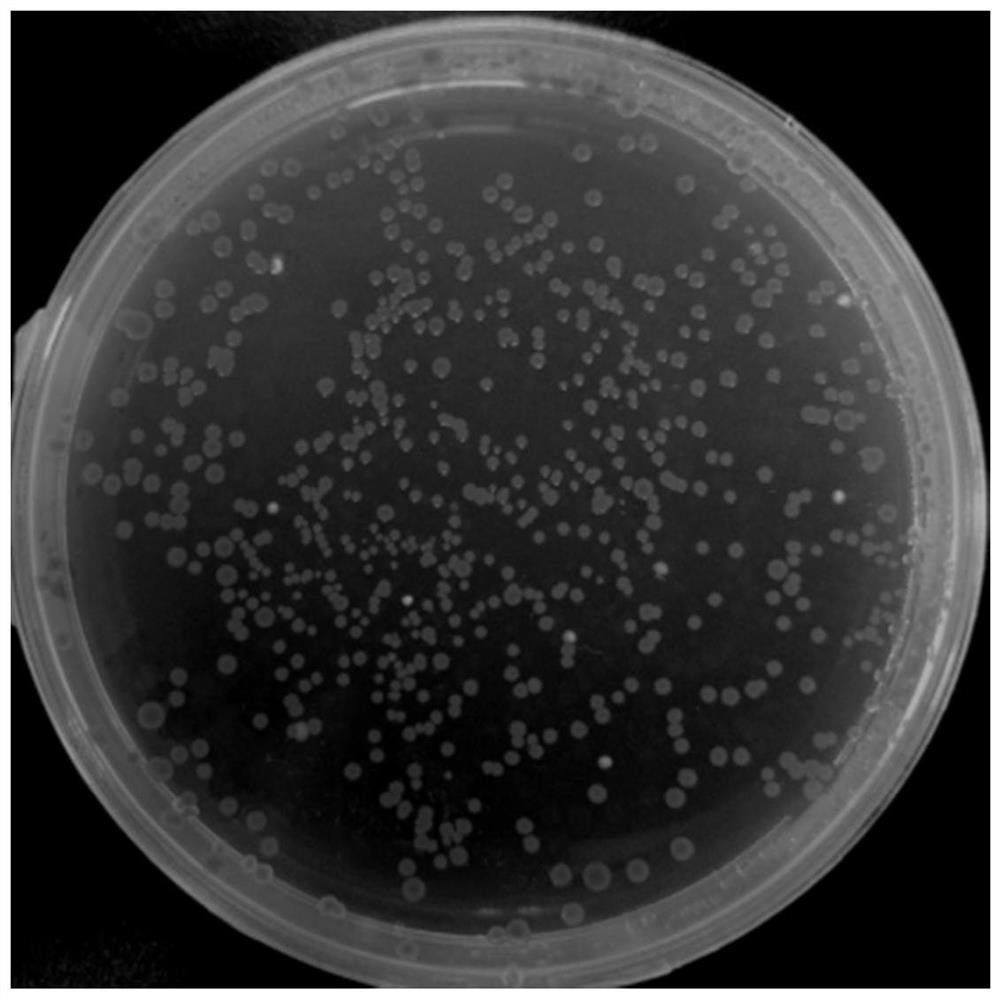
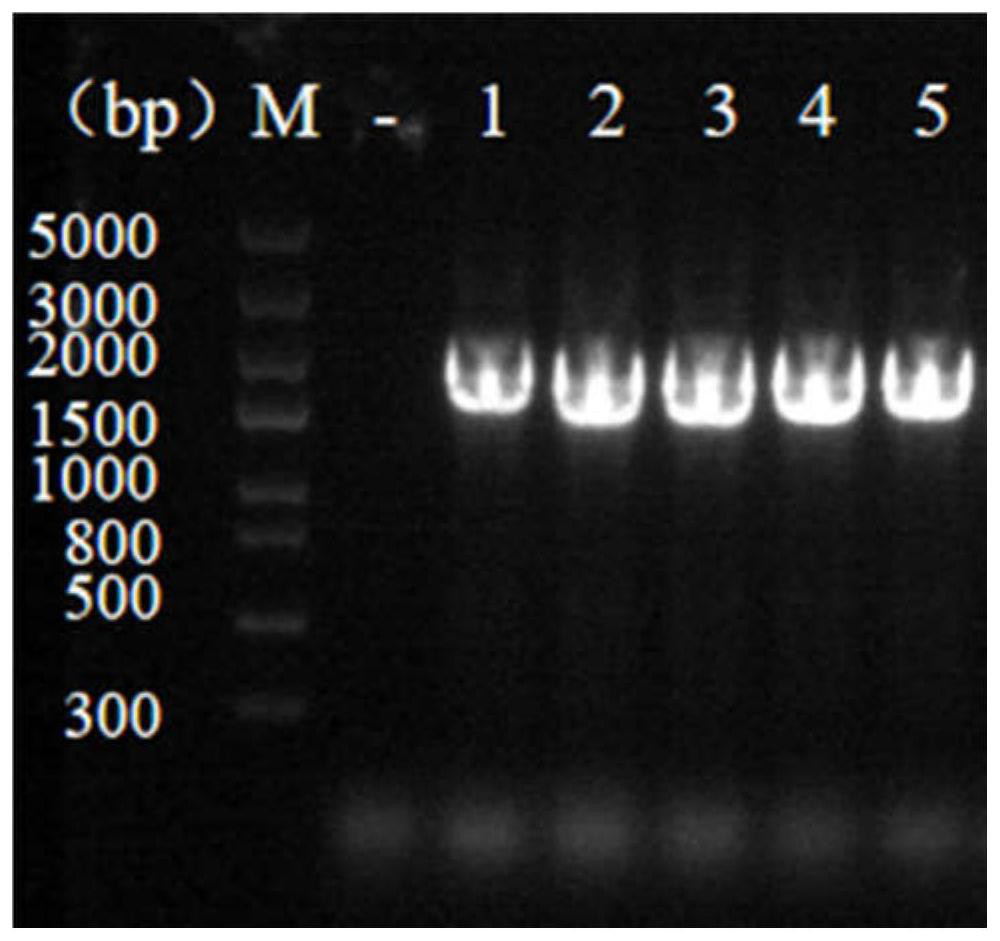
[0046] The recombinant plasmid HBM-flag-RBD-hFc_pfastBac1 was transformed into Escherichia coli DH10Bac competent cells, and the blue-white spot screening recombinants were carried out on the LB plate containing X-Gal / IPTG ( figure 2 ), select 5 white colonies for PCR identification, the target fragment size is 1650bp, and the identification results are as fo...
Embodiment 2
[0077] Extraction and purification of new coronavirus RBD fusion protein subunit vaccine insect rod system:
[0078] (1) Sample pretreatment:
[0079] Centrifuge the new coronavirus RBD fusion protein subunit vaccine insect rod system and add imidazole to 500ml of culture supernatant after centrifugation to a final concentration of 30mM. After adding, fully stir until completely dissolved;
[0080] Use NaCl to adjust the conductance to 50ms / cm, adjust the pH to 6.2, filter with a 0.45um membrane, and use the filtrate for chromatographic purification;
[0081] (2) Affinity chromatography
[0082] (1) The affinity chromatography filler is Ni filler resistant to EDTA and alkali; the imidazole concentration, conductivity and pH value of the filler equilibrium solution are the same as the pretreatment solution: 40mM PB, NaCl concentration 0.6M, imidazole concentration 30mM, pH6.2 equilibrium; The height is 15cm, the flow rate is 300cm / h; the equilibrium volume is 20CV;
[0083] ...
Embodiment 3
[0091] Extraction and purification of new coronavirus RBD fusion protein subunit vaccine insect rod system:
[0092] (1) Sample pretreatment:
[0093] Centrifuge the new coronavirus RBD fusion protein subunit vaccine insect rod system and add imidazole to 500ml culture supernatant after centrifugation to a final concentration of 5mM. After adding, stir well until completely dissolved;
[0094] The above treatment solution was adjusted with NaCl to conductance to 20ms / cm, pH to 8.0, filtered with a 0.45um membrane, and the filtrate was used for chromatographic purification.
[0095] (2) Affinity chromatography
[0096] (1) The affinity chromatography filler is Ni filler resistant to EDTA and alkali; the imidazole concentration, conductivity and pH value of the filler equilibrium solution are the same as the pretreatment solution: 0.1M PB, NaCl concentration 0.2M, imidazole concentration 5mM, pH8.0 balance; The column height is 10cm, the flow rate is 100cm / h; the equilibrium vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com