Anti-cancer drug-combination composition comprising compound F-A
An anti-cancer drug and a combined drug technology, applied in the field of medical use of flavonoids, can solve the problems of myocardial necrosis, no solution, congestive heart failure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The therapeutic effect of embodiment 1 compound F-A on doxorubicin-induced cardiotoxicity
[0037] Rabbit test method for doxorubicin-induced cardiomyopathy (this experiment complies with relevant ethical regulations):
[0038] (1) Test with two sexes of rabbits whose initial body weight is 2.3 ± 0.2kg, group a is untreated animals (for control animals, n=8), group b is animals treated with doxorubicin (+ replace test substance with placebo, n=8), group c is doxorubicin and test substance treated animals (n=8), test substance is flavonoids F-A, F-B, F-C, respectively c1, c2 , Group c3.
[0039] (2) Groups b and c administered intravenously twice a week with doxorubicin, 1 mg / kg each time, for a total of 4 weeks, and group c took orally administered the test substance 20 mg / kg body weight per day, starting from the first day of doxorubicin treatment To start, feed the feed at the same time.
[0040] (3) After 4 weeks, separate the heart and weigh it, and detect the co...
Embodiment 2
[0047] Therapeutic Effect of Example 2 Compound F-A on Doxorubicin-induced Cardiotoxicity
[0048] Inhibition of cardiomyocyte apoptosis in the H9c2 cardiotoxicity model:
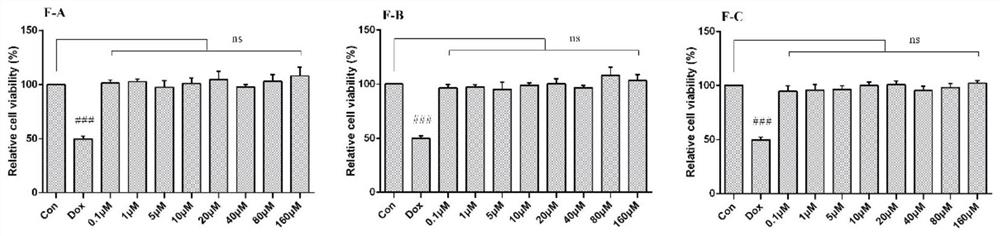
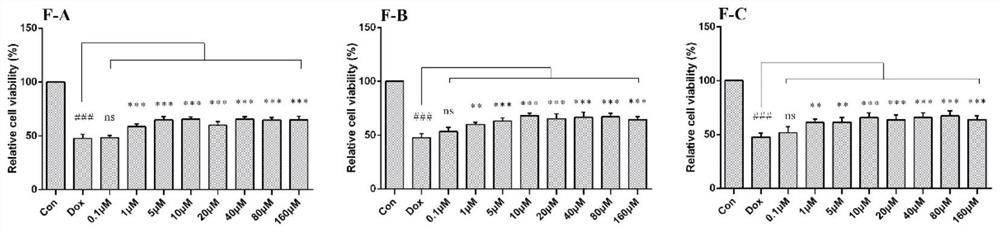
[0049](1) After incubating H9c2 cells with different concentrations of flavonoids F-A, F-B, and F-C for 24 hours, the toxic effects of flavonoids on cardiomyocytes were evaluated by MTT colorimetry. H9c2 cells were incubated with flavonoids for 1 hour before adding 2.5 After cultured with μM DOX for 24 hours, MTT colorimetric method was used to screen the activity of flavonoids on doxorubicin (DOX)-induced cardiomyocyte apoptosis, and determine the optimal concentration range.
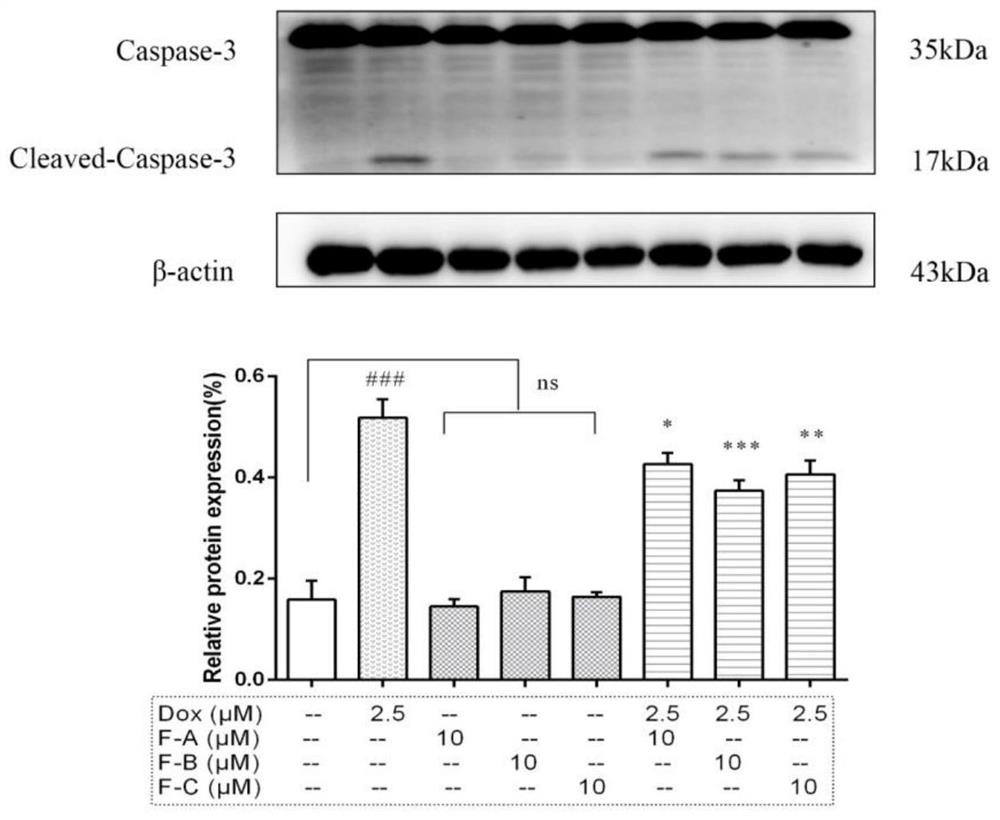
[0050] (2) After treating H9c2 cardiomyocytes with different seabuckthorn flavonoids for 1 hour, treat them with 2.5 μM DOX for 24 hours, then extract the protein, lyse, scrape, collect the cell lysate, centrifuge at 12000 g for 15 minutes, collect the supernatant and discard the precipitate, - Store at 80°C, and use BCA method to ...
Embodiment 3
[0057] Embodiment 3 contains the tablet of flavonoid compound
[0058] The following ingredients are produced for each tablet:
[0059]
[0060] The above-mentioned components were mixed according to the stated dosage, granulated, blended and compressed into tablets to prepare 250 mg tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com