Method for catalytically synthesizing lactide
A technology for lactide and compound, applied in the field of catalyzing and synthesizing lactide, can solve the problems of high catalytic reaction temperature and large energy consumption, and achieve the effects of low reaction temperature, high yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
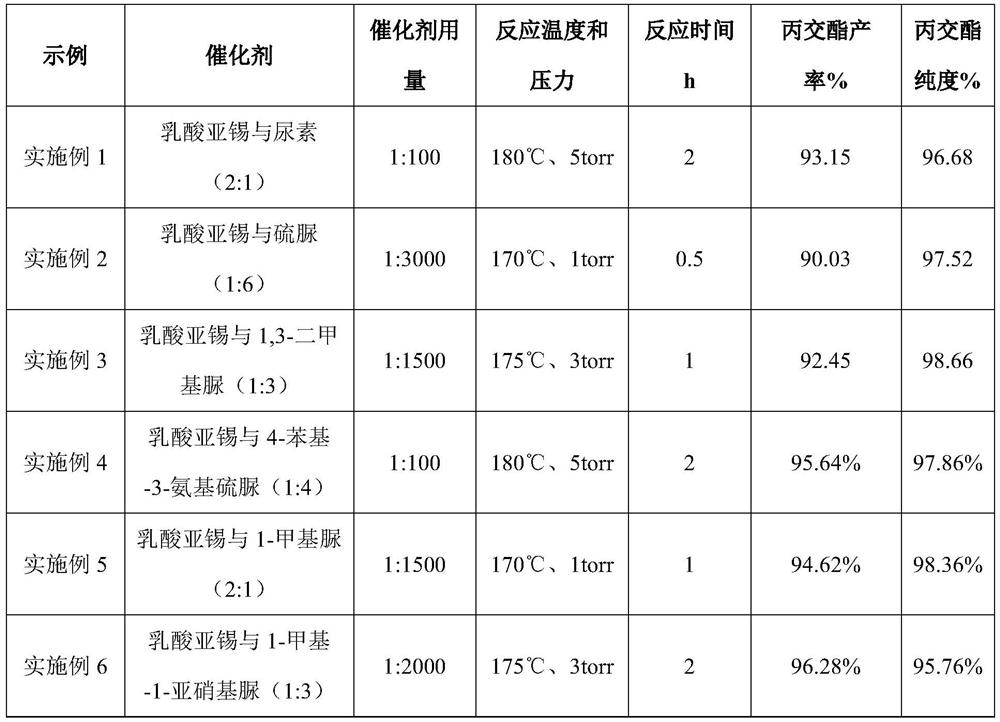
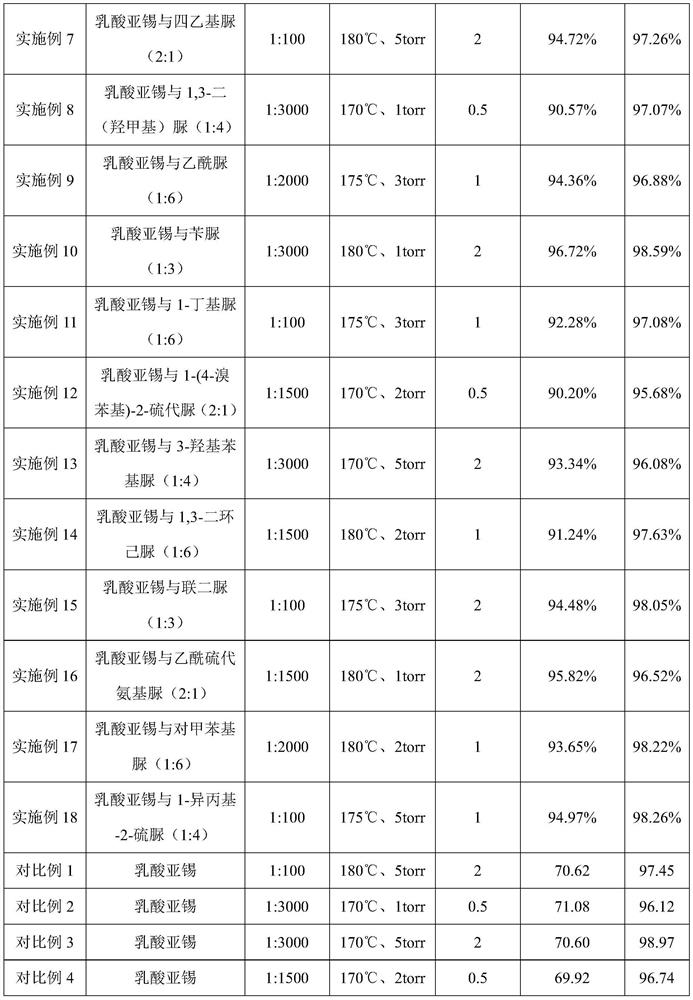
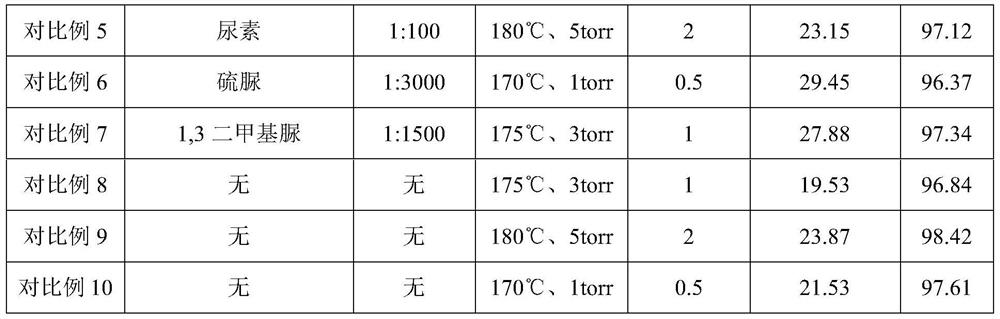
[0024] 1) Mix stannous lactate and urea at a molar ratio of 2:1, heat and stir at 80°C and 500r / min to form a composite catalyst;
[0025] 2) Add 150 g of L-lactic acid oligomers with a weight-average molecular weight of about 500 Da to the reaction flask, and add the composite catalyst prepared in step (1) into the lactic acid oligomers. The ratio is 1:100, the reaction temperature is controlled at 180°C and the vacuum degree is 5 torr, and the reaction is carried out for 2 hours. The yield of L-lactide is 93.15%, and the purity is 96.68%.
Embodiment 2
[0027] 1) Mix stannous lactate and thiourea at a molar ratio of 1:6, heat and stir at 90°C and 100r / min to form a composite catalyst;
[0028] 2) Add 150 g of L-lactic acid oligomers with a weight-average molecular weight of about 3000 Da to the reaction flask, and add the composite catalyst prepared in step (1) into the lactic acid oligomers. The ratio is 1:3000, the reaction temperature is controlled at 170°C and the vacuum degree is 1 torr, and the reaction is carried out for 0.5 hours. The yield of L-lactide is 90.03%, and the purity is 97.52%.
Embodiment 3
[0030] 1) Mix stannous lactate and 1,3-dimethylurea at a molar ratio of 1:3, heat and stir at 80°C and 300r / min to form a composite catalyst;
[0031] 2) Add 150 g of L-lactic acid oligomers with a weight-average molecular weight of about 1500 Da to the reaction flask, and add the composite catalyst prepared in step (1) into the lactic acid oligomers. The ratio is 1:1500, the reaction temperature is controlled at 175°C and the vacuum degree is 3 torr, and the reaction is carried out for 1 hour. The yield of L-lactide is 92.45%, and the purity is 98.66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com