Oxadiazolopyrazines and oxadiazolopyridines useful as mitochondrial uncouplers
An alkyl, C2-C8 technology, applied in the field of oxadiazolopyrazine and oxadiazolopyridine that can be used as mitochondrial uncoupling agent, can solve the problem of drugs without uncoupling agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
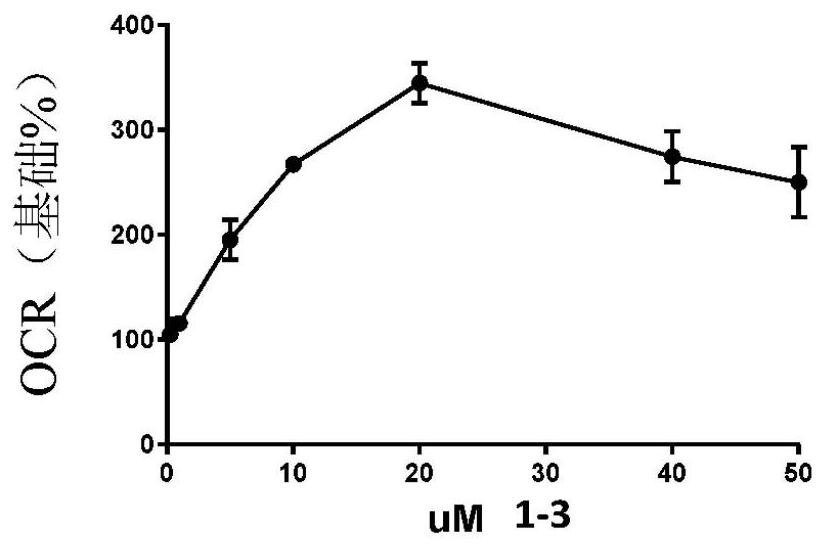
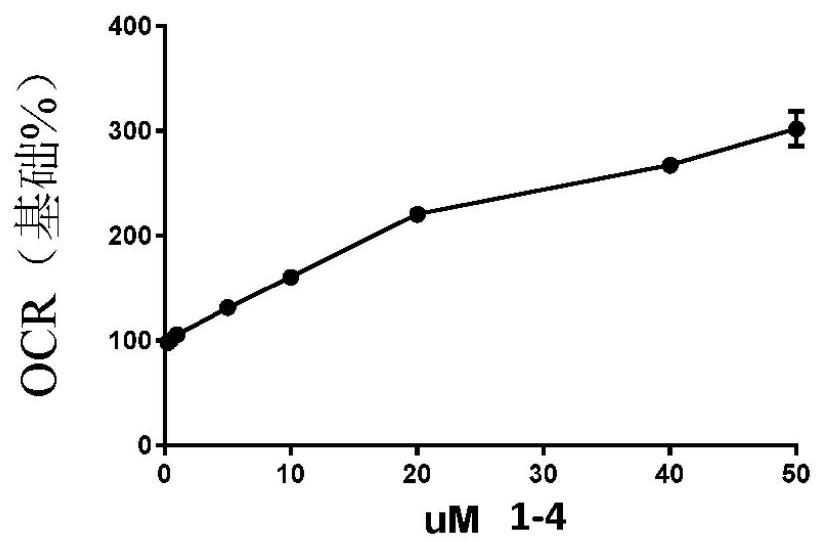
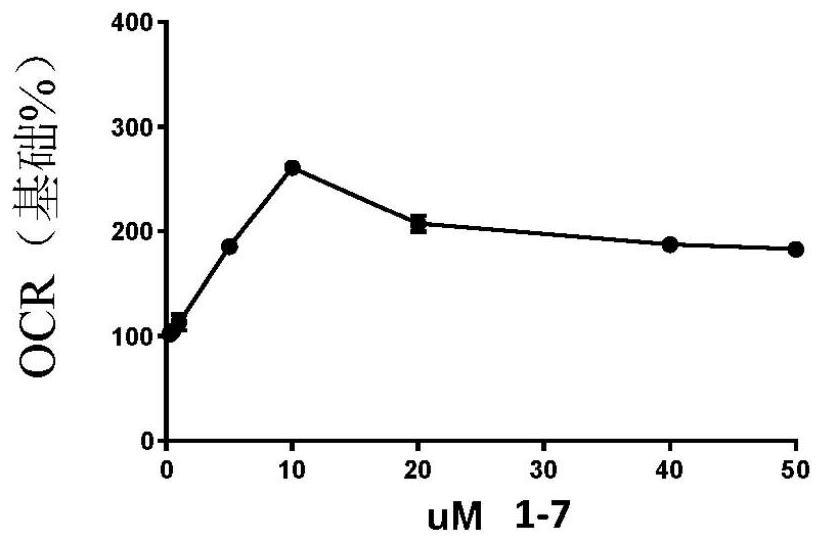
Image
Examples
example
[0211] general method
[0212] The following starting materials and general procedures were used in the following synthetic examples.
[0213] In all synthetic examples, room temperature (rt) was about 21 °C.
[0214] NMR solvent reference: i (CD 3 ) 2 CO (2.05 / 29.84ppm); (CD 3 ) 2 SO (2.50 / 39.52ppm).
[0215] NMR Abbreviations: aq. = aqueous, app = apparent, br = broad, s = singlet, d = doublet, t = triplet, q = quartet, p = quintet. * refers to rotamers.
[0216] Synthesis of starting materials
example 1
[0217] Example 1. Synthesis of [1,2,5]oxadiazolo[3,4-B]pyrazine-5,6-diol (1-1)
[0218]
[0219] In a 500 mL round bottom flask equipped with a condenser, 1,2,5-oxadiazole-3,4-diamine (50.0 g, 500 mmol) and oxalic acid (49.6 g, 551 mmol) were dissolved in aqueous HCl (250 mL, 10 The mixture in % v / v) was heated to reflux in a sand bath for 4 hours. The resulting mixture was cooled in an ice bath, and the precipitate was filtered, rinsed with water (20 mL) followed by diethyl ether (2 x 150 mL), and collected to give 1-1 (55.2 g, 72%) as a colorless solid : 1 H NMR ((CD 3 ) 2 CO,400MHz)δ11.65(brs,2H); 13 C NMR ((CD 3 ) 2 CO, 100MHz) δ153.9, 144.8.
example 2
[0220] Synthesis of Example 2.5,6-dichloro-[1,2,5]oxadiazolo[3,4-B]pyrazine (1-2)
[0221]
[0222] With a glass stopper and with aqueous Na 2 CO 3 Diol 1-1 (37.0 g, 240 mmol) and PCl 5 (120g, 576mmol) in POCl 3 (45 mL) was heated to 95 °C for 2 hours. The mixture was allowed to cool to room temperature, and then to 5-10 °C in an ice bath. The reaction mixture was slowly poured into ice-cold water (3 × 250 mL beakers, each with 100 mL H 2 O) so that the temperature of the water does not rise above 25°C (monitored by a thermometer in the water bath). The colorless precipitate was filtered, rinsed with water, and dissolved in acetone (about 200 mL). Water (3 times the volume of acetone) was added to the organic solution to facilitate precipitation, and the colorless solid was collected by filtration under vacuum with P 2 o 5 Drying as a desiccant afforded 1-2 (35.1 g, 77%) as a colorless solid: 13 C NMR ((CD 3 ) 2 CO, 100MHz) δ155.9, 151.9.
[0223] The large-scal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com