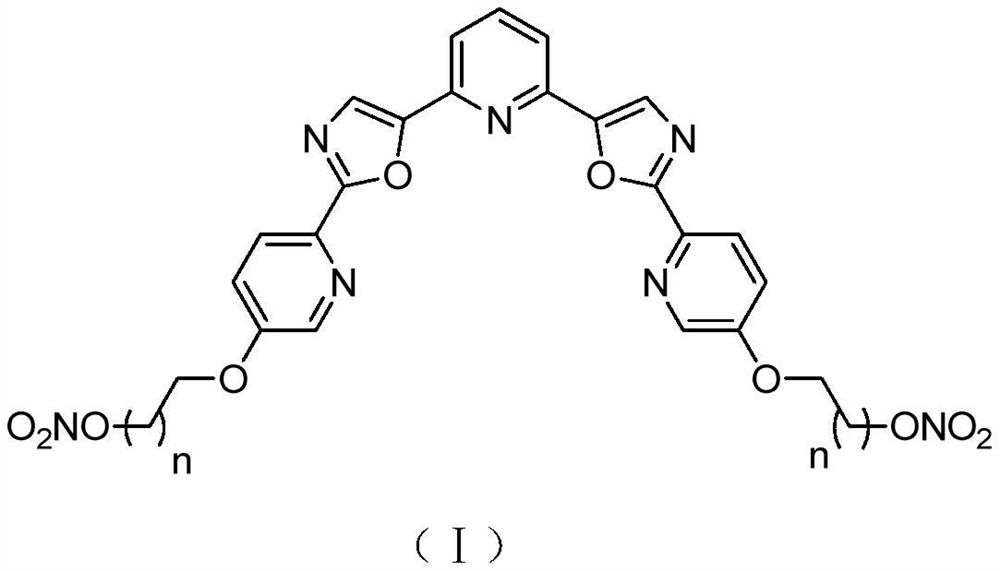
Nitrate NO donor type tripyridine bisoxazole compound as well as preparation method and application thereof
A technology of tripyridine bioxazole and nitrate, applied in digestive system, organic chemistry, drug combination and other directions, can solve the problems of poor selectivity, strong toxic and side effects, and achieve the effects of high selectivity, high toxic and side effects, and weak toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of compound 1:
[0028]
[0029] Dissolve 2,6-pyridinedicarbaldehyde (14.6mmol) in 60mL of methanol, add 2 times the amount of TosMIC (24.8mmol) and 4 times the amount of K 2 CO 3 (58.4mmol), reflux reaction for 5 hours, after the reaction was finished, the solvent was evaporated, washed with water, filtered, and purified by silica gel column chromatography (petroleum ether / ethyl acetate=40:1) to obtain 2.7g yellow solid with a yield of 87%. . 1 H NMR (400MHz, CDCl 3 )8.01(s, 2H), 7.80(t, J=8.7Hz, 1H), 7.63(s, 2H), 7.56(d, J=8.6Hz, 2H). ESI-MS: m / z 214.1 [M+ H] + .
Embodiment 2
[0031] Synthesis of compound 3:
[0032]
[0033] Dissolve 4.7mmol of Intermediate 1 in 20mL of dioxane, add 3.3 times the amount of 5-bromo-2methoxypyridine (15.5mmol), and then add 0.4 times the amount of Pd(OAC) in a stirring state after dissolution 2 (1.9mmol), 4.4 times the amount of CsCO 3 (20.6mmol), 4.4 times the amount of CuI (10.8mmol) and 0.2 times the amount of PCy 3 .HBF 4 (0.94mmol), reacted at 130°C for 24 hours under the protection of argon, evaporated the solvent to obtain the black crude product 2, was dissolved in 120mL of dichloromethane after drying, N 2 Slowly drop 3 times the molar amount of BBr in a low-temperature reactor at -25°C under protective conditions 3 / CHCl 2 , slowly return to room temperature after the dropwise addition, and continue to stir for 24 hours. After the reaction is completed, the reaction system is quenched with methanol and water successively, dichloromethane is evaporated, and silica gel column chromatography (petroleum ...
Embodiment 3
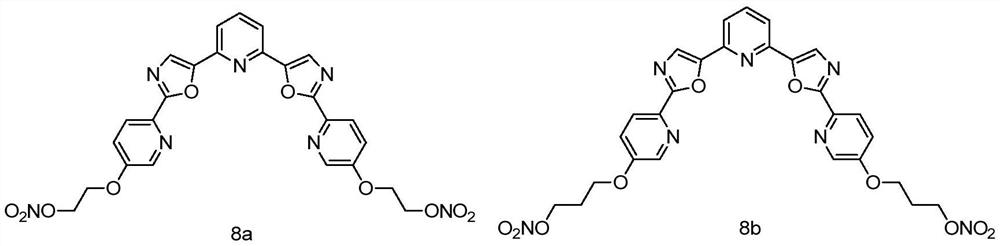
[0035] Synthesis of Compound 8a:
[0036]
[0037] Concentrated sulfuric acid (20mmol) was added dropwise to fuming nitric acid (20mmol) under ice-cooling, and after stirring for 10min, a dichloromethane solution of bromoethanol (10mmol) was added dropwise. After reacting for 3 h, 10 mL of water was added to terminate the reaction. The organic phase was separated, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain compound 6, which was directly used in the next reaction.
[0038] Compound 3 (0.5mmol) in Example 2 was placed in a reaction flask, DMF (8mL) was added, stirred until the compound was completely dissolved, DBU (0.75mmol) and 6 (0.75mmol) were added to react at room temperature for 5 hours, and the reaction was monitored by TLC process, after the reaction is over, pour the reaction solution into 50ml of ice-water mixture, extract three times with 100ml of dichloromethane, wash with saturated brine, dry over anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com