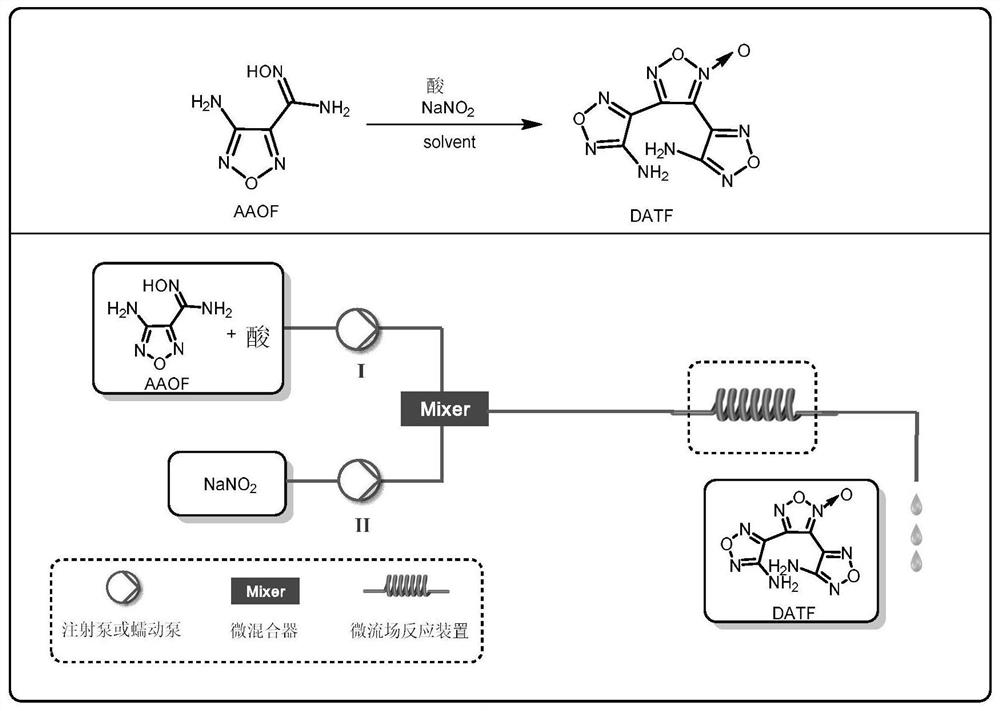
A method for one-step synthesis of 3,4-bis(4'-aminofurazan-3'-yl)oxyfurazan
A technology of aminofurazan and furoxan, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve problems such as high industrial production costs, large equipment footprint, and uncontrollable safety, and achieve Stable product quality, small footprint, good material mixing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
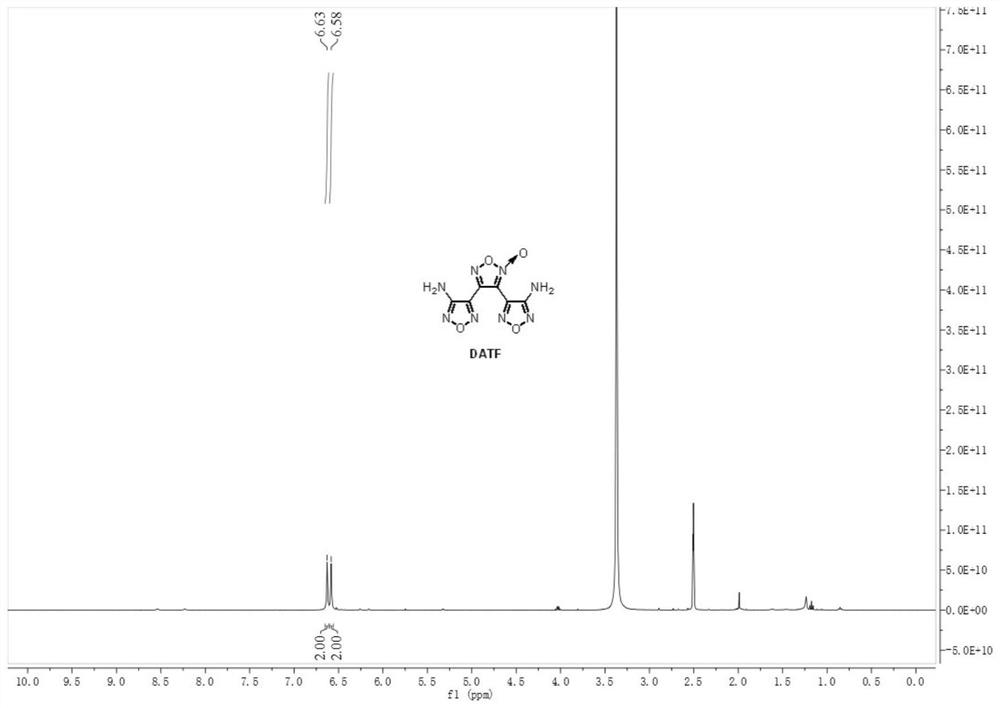
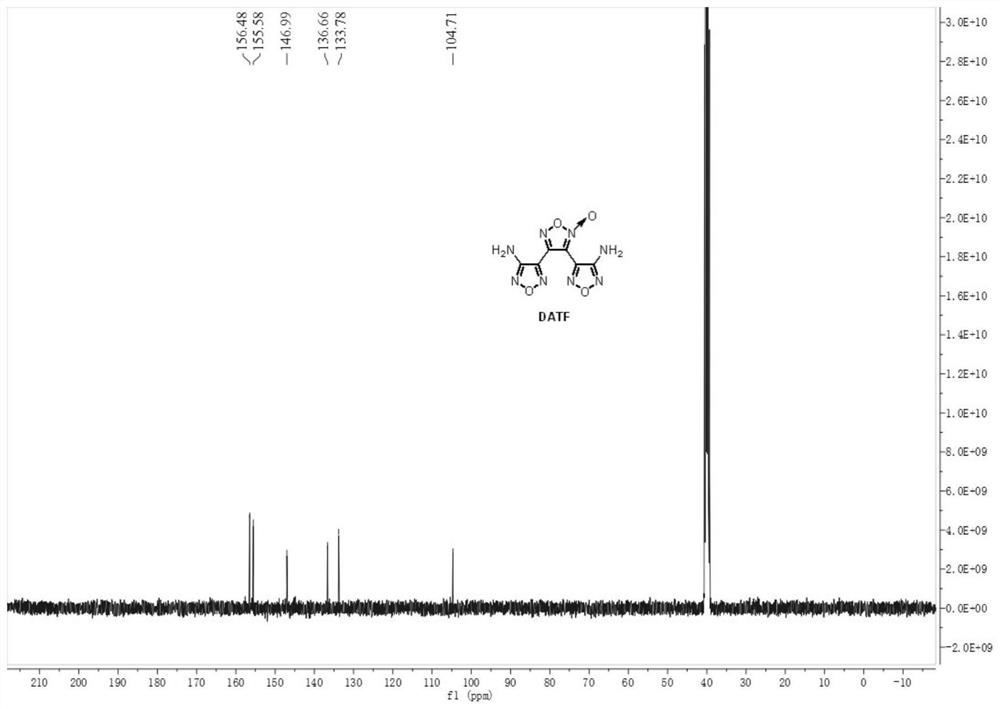
[0044] Weigh 0.143 g (1.0 mmol, 1.0 equiv) of 3-amino-4-amidoxime furoxan (AAOF), add it to acetonitrile for dissolving, add 286 μL acetic acid (5.0 mmol, 5.0 equiv) to prepare a 4.5 mL solution, As material Ⅰ; weigh 0.069gNaNO 2 (1.0mmol, 1.0equiv) was added into water to prepare a 1.5mL solution as material Ⅱ. The feed pump simultaneously pumps in material I and material II, mixes them in the mixer, and transports them to a microchannel reactor (inner diameter 0.6mm, length 7m, volume 2mL) for reaction at a reaction temperature of 25°C. Among them, the flow rate of material I is 0.2mL / min, the flow rate of material II is 0.066mL / min, and the reaction residence time is 7.5min. The reaction effluent was collected and analyzed by high-performance liquid phase detection. The raw material conversion rate was 80.7%, and the product yield was 47.1%. figure 1 and figure 2 shown.
Embodiment 2
[0046]Weigh 0.143g (1.0mmol, 1.0equiv) of 3-amino-4-amidoxime furoxan (AAOF), add to acetonitrile to dissolve, add 278μL concentrated sulfuric acid (5.0mmol, 5.0equiv) to prepare a 4.5mL solution , as material Ⅰ; weigh 0.069gNaNO 2 (1.0mmol, 1.0equiv) was added into water to prepare a 1.5mL solution as material Ⅱ. The feed pump simultaneously pumps in material I and material II, mixes them in the mixer, and transports them to a microchannel reactor (inner diameter 0.6mm, length 7m, volume 2mL) for reaction at a reaction temperature of 25°C. Among them, the flow rate of material I is 0.2mL / min, the flow rate of material II is 0.066mL / min, and the reaction residence time is 7.5min. The reaction effluent was collected and analyzed by high performance liquid phase detection. The conversion rate of the raw material was 86.7%, and the yield of the product was 54.1%.
Embodiment 3
[0048] Weigh 0.143g (1.0mmol, 1.0equiv) of 3-amino-4-amidoxime furoxan (AAOF), add to acetonitrile to dissolve, add 333μL concentrated nitric acid (5.0mmol, 5.0equiv) to prepare a 4.5mL solution , as material Ⅰ; weigh 0.069gNaNO 2 (1.0mmol, 1.0equiv) was added into water to prepare a 1.5mL solution as material Ⅱ. The feed pump simultaneously pumps in material I and material II, mixes them in the mixer, and transports them to a microchannel reactor (inner diameter 0.6mm, length 7m, volume 2mL) for reaction at a reaction temperature of 25°C. Among them, the flow rate of material I is 0.2mL / min, the flow rate of material II is 0.066mL / min, and the reaction residence time is 7.5min. The reaction effluent was collected and analyzed by high performance liquid phase detection. The conversion rate of the raw material was 82.6%, and the yield of the product was 50.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com