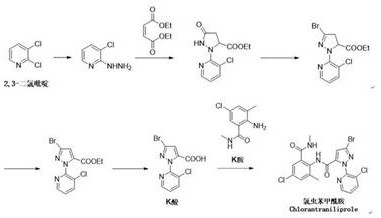
Preparation method of key intermediate K acid of chlorantraniliprole
A technology of chlorantraniliprole and intermediates, which is applied in the field of preparation of K acid, the key intermediate of chlorantraniliprole, can solve the problems of multiple impurities, low yield, harsh condition control, etc., and achieve good selectivity, The effect of high product purity and high ring-forming yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
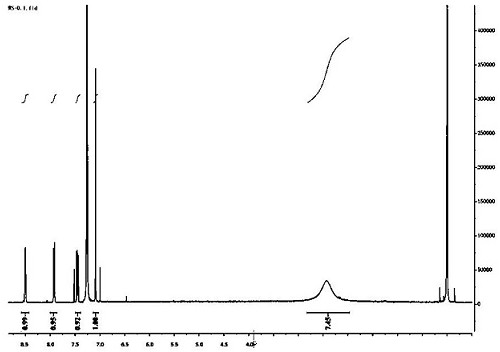
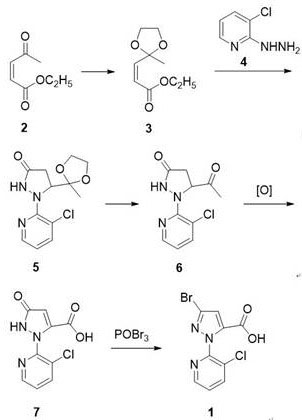
[0012] A kind of preparation method of chlorantraniliprole key intermediate K acid:
[0013] 1. Add 142g (1mol) of ethyl acetylacrylate, 93g (1.5mol) of ethylene glycol, and 19g (0.1mol) of p-toluenesulfonic acid (containing a part of crystal water) to 350ml of toluene, The device was heated to reflux, and the dehydration reaction was about 6h. The gas chromatography detected that the reaction raw materials disappeared. After the reaction, cool to below 10°C, wash with 200ml×2 water and 200ml saturated brine, separate the organic layer, add sodium bicarbonate to dry, filter, wash the filter cake with a small amount of toluene, and combine several layers. Distilled under reduced pressure, evaporated to substantially constant weight, 179g of light yellow oily matter, the gas chromatography detection purity of this oily matter is about 95%, can be directly used in the next step reaction without further separation.
[0014] 2. Dissolve the above yellow oil in 450ml of absolute et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com