A kind of intermediate of betrixaban and its preparation method and application
A betrixaban maleate and reaction technology, applied in the intermediates of betrixaban and preparation thereof, benzoxazinone compounds and the field of preparation thereof, can solve the problem of not effectively shortening the synthesis route of betrixaban , Commercial sources are not easy to obtain, poor stability of starting materials, etc., to achieve the effects of cheap raw materials, shortening the synthesis route, and reducing synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
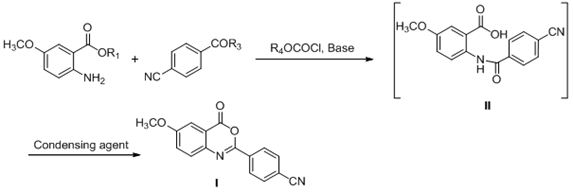
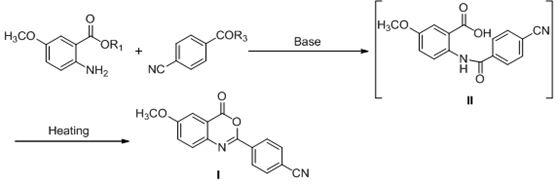
[0072] Example 1: Preparation of 2-(4-cyanophenyl)-6-methoxybenzo[d]-1,3-oxazin-4-one (I)
[0073]
[0074] Add 5.0g of 5-methoxy-2-aminobenzoic acid (29.9mmol) and 25mL of dichloromethane into a 150mL reaction flask, stir to dissolve and add 5.0mL of triethylamine (TEA) (36.1mmol). The mixture was cooled to 0-10°C, 5.9 g of 4-cyanobenzoyl chloride (35.6 mmol) in toluene (25 mL) was added dropwise to the mixture, and the reaction solution was warmed to room temperature and stirred for 8 hours. After the reaction of 5-methoxy-2-aminobenzoic acid was detected by TLC, 35 mL of purified water was added to wash the reaction solution. The organic layer was separated, heated to the reflux temperature of toluene and stirred for 2 hours. After the completion of the reaction as detected by TLC, the reaction solution was slowly cooled to 0-10° C., and the precipitated solid was filtered, washed and dried to obtain 7.36 g of compound I with a yield of 88.6% and a purity of 99.8%. 1 H...
Embodiment 2
[0075] Example 2: Preparation of 2-(4-cyanophenyl)-6-methoxybenzo[d]-1,3-oxazin-4-one (I)
[0076]
[0077] Add 2.0g of 5-methoxy-2-aminobenzoic acid (12.0mmol), 10mL of dichloromethane and 1mL of N-methylmorpholine (NMM) into a 100mL reaction flask, stir to dissolve and cool to 0-10°C. 1.76 g of 4-cyanobenzoic acid (12.0 mmol) and 1.8 g of isobutyl chloroformate were added to the mixture. The reaction solution was warmed up to room temperature and stirred for 5 hours. TLC detects that after the reaction of 5-methoxy-2-aminobenzoic acid is completed, the reaction solution is 35-45 ° C, concentrated to dryness under reduced pressure, and 10 mL of carbon tetrachloride (CCl 4 ) and 3.46g triphenylphosphine (13.2mmol), reacted at 30-35°C and stirred for 5 hours. After the reaction was detected by TLC, 50mL of n-hexane was added to the reaction solution, and the suspended matter was precipitated and stirred for 30 minutes, filtered, and the solid was dried 2.84 g of compound I...
Embodiment 3
[0078] Example 3: Preparation of 2-(4-cyanophenyl)-6-methoxybenzo[d]-1,3-oxazin-4-one (I)
[0079]
[0080] Add 2.0 g of 5-methoxy-2-aminobenzoic acid (12.0 mmol), 10 mL of dichloromethane and 5.1 g of 1-ethyl-(3-dimethylaminopropyl)carbodiene to a 100 mL reaction flask Amine hydrochloride (EDC) (26.4mmol), stirring to dissolve and cooling to 0-10°C, adding 1.76g 4-cyanobenzoic acid (12.0mmol) and 1.9g 1-hydroxybenzotriazole to the mixture (14.4 mmol) (HOBT). The reaction solution was warmed to room temperature and stirred for 12 hours. After the reaction of 5-methoxy-2-aminobenzoic acid was detected by TLC, 50 mL of n-hexane was added to the reaction solution, and the suspended matter was precipitated and stirred for 30 minutes, filtered, and the solid was dried to obtain 2.83 g of compound I, with a yield of 84.8%. The purity is 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com