Application of compound as polyimide curing accelerator, polyimide precursor as well as preparation method and application of polyimide precursor
A technology of polyimide precursor and curing accelerator, applied in the field of polyimide materials, can solve the problems of curing agent residue, large amount of addition, continuous volatilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
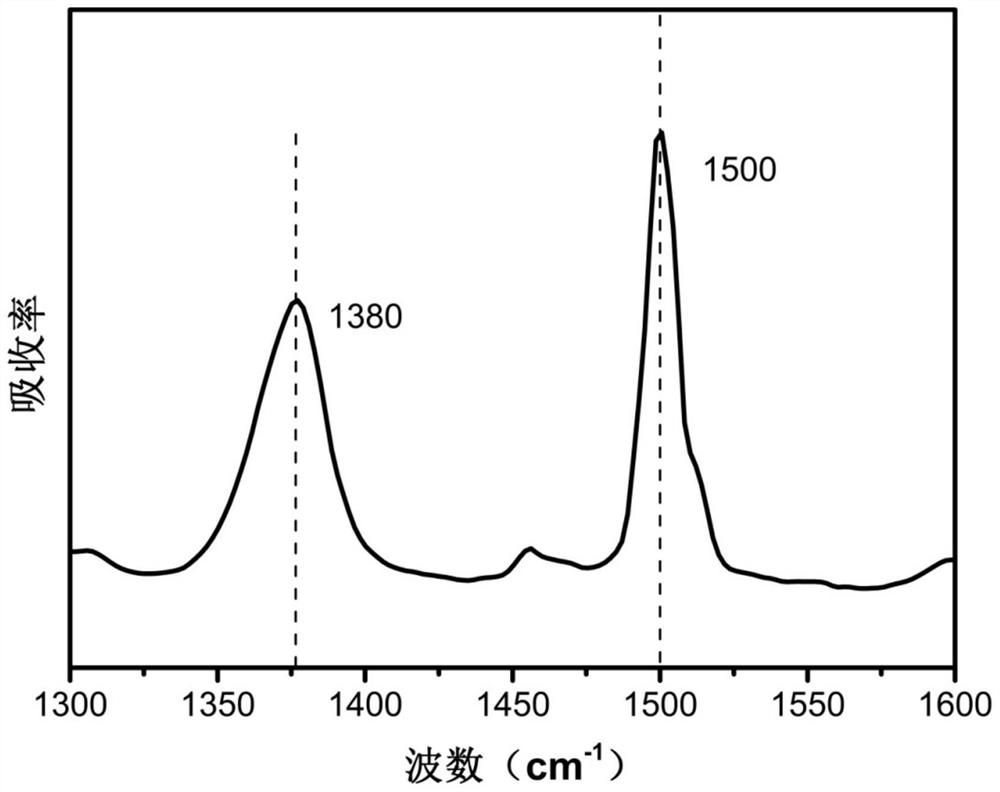
[0069] This embodiment provides a polyimide precursor and a polyimide film, and the preparation method is as follows:
[0070] (1) At room temperature, under an inert atmosphere and mechanical stirring, dissolve 1.99g of 4,4'-diaminodiphenyl ether in 37.53g of N-methylpyrrolidone solvent, and wait until the 4,4'-diaminodiphenyl ether is completely After dissolving, divide 2.18g of 1,2,4,5-pyromellitic dianhydride into two equal parts, add them to the diamine solution in batches at intervals of 20 minutes, and stir mechanically for 6 hours to obtain a polyimide precursor solution .
[0071] (2) At room temperature, under an inert atmosphere and mechanical stirring, 55.08 mg of curing accelerator 5-aminobenzimidazole (accounting for 4.14% by mole of the dianhydride monomer) is added to the polyimide obtained in step (1) In the precursor solution, react for 2h. Then place it in a vacuum drying oven, defoaming for 2h, to obtain a 5-aminobenzimidazole-polyimide precursor solution...
Embodiment 2
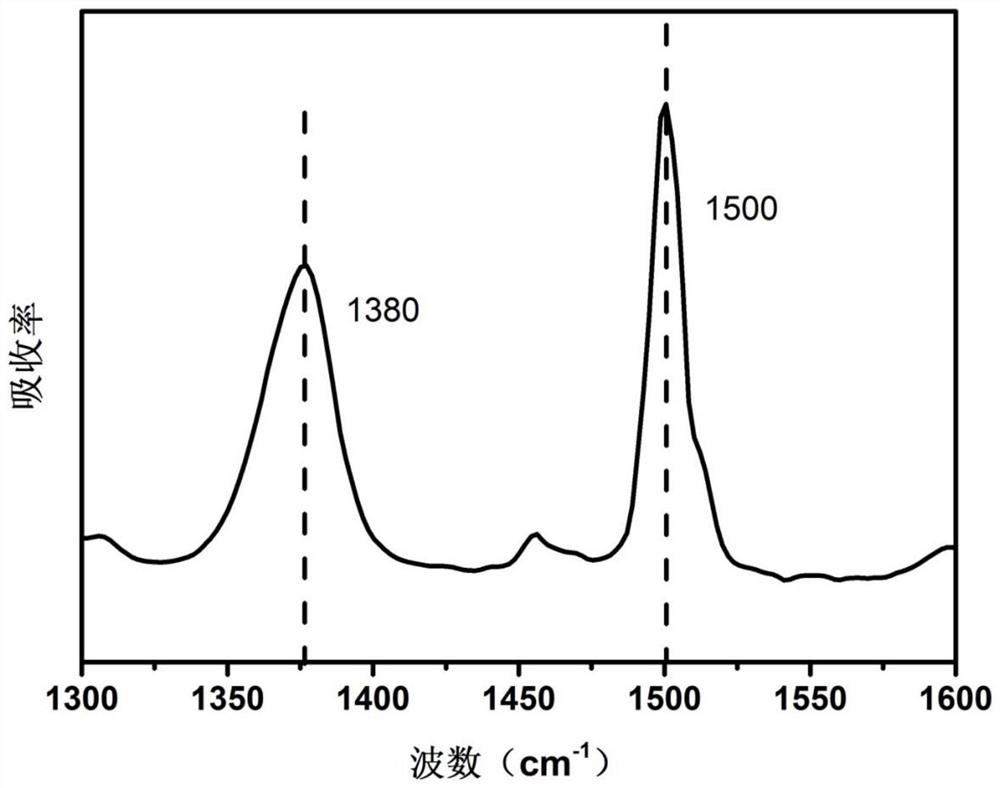
[0075] This example provides a polyimide precursor and a polyimide film. The difference between the preparation method and Example 1 is that the N-methylpyrrolidone in step (1) is replaced by the same quality N,N-di Methyl acetamide to obtain 5-aminobenzimidazole-polyimide film.
Embodiment 3
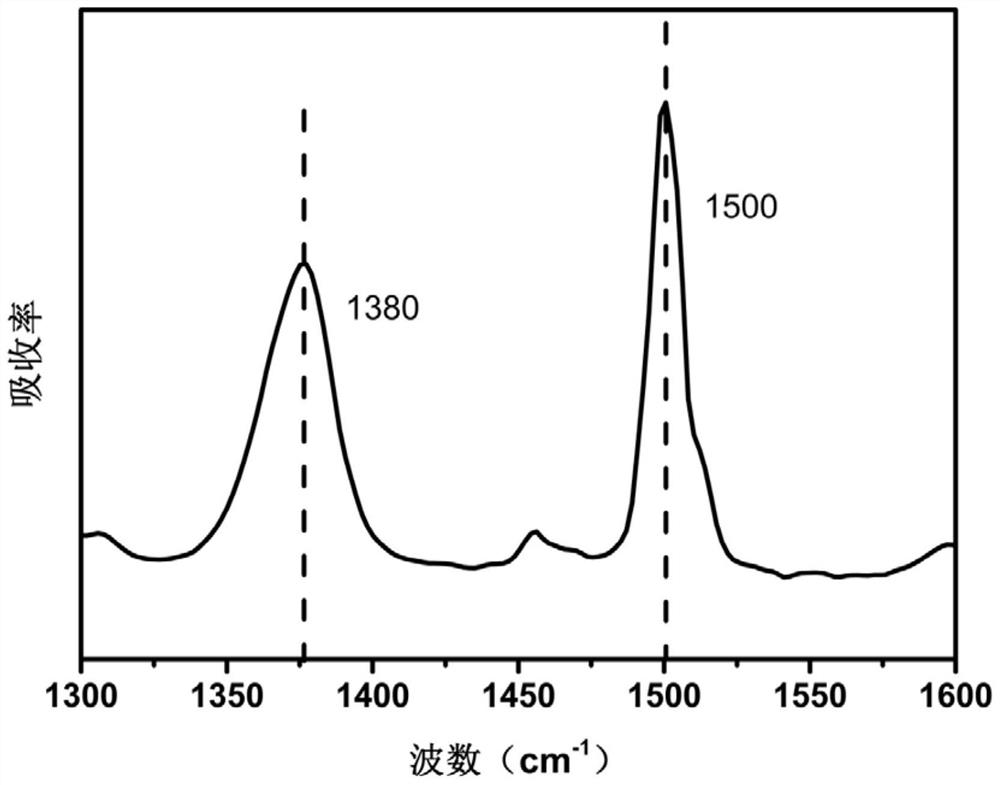
[0077]This example provides a polyimide precursor and a polyimide film. The difference between the preparation method and Example 1 is that the N-methylpyrrolidone in step (1) is replaced by N,N-dimethylformaldehyde Amide to obtain 5-aminobenzimidazole-polyimide film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com